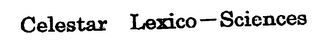Information
-
 Trademark
Trademark
-
 76248470
76248470
-
Serial Number
76248470
-
Registration Number
2907497
-
International Classifications
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
- 42 - Scientific and technological services and research and design relating thereto
-
Filing Date
April 25, 2001
23 years ago
-
Registration Date
December 07, 2004
19 years ago
-
Transaction Date
August 16, 2011
12 years ago
-
Status Date
July 08, 2011
13 years ago
-
Published for Opposition Date
September 14, 2004
19 years ago
-
Location Date
December 07, 2004
19 years ago
-
Status Code
710
-
Current Location
PUBLICATION AND ISSUE SECTION
Employee Name
CARRUTHERS, ALICE S
-
Attorney Docket Number
TM01_083/KRO
Attorney Name
Kaushal R. Odedra
Law Office Assigned Location Code
L80
-
Owners
Mark Drawing Code
1000
Mark Identification
CELESTAR LEXICO-SCIENCES
Case File Statements
- GS0091: Computer programs for use in analyzing and visualizing nucleic acid, protein, and phage information and expression; for use in database management, integration and manipulation; for use in characterization of nucleic acid and protein information; for use in analyzing chemical structure and activity information; for use in acquisition and interrogation of clinical, pathological, diagnostic, genetic, genomic and biochemical data; for use in clinical trial design and management; for use in text and pattern recognition; for storage, retrieval and analysis of life sciences literature; for use in managing robot function; for use in processing toxicological and pharmacological information; for laboratory instrument control; for use in control of sample input to mass spectrometers; for use in spectral analysis; prerecorded CD-ROMs featuring databases containing life sciences information; pre-configured microprocessors for analysis and storage of life sciences information; computer programs for use in the management, storage, retrieval, and analysis for data in the discovery and development of diagnostic, prognostic, and therapeutic medical products; for use in identifying and characterizing proteins; for use in controlling mass spectrometers; for use in spectral analysis; for use in controlling affinity capture optical biosensors; for use in analyzing and characterizing plasmon resonance; for use in controlling liquid chromatography instruments; for use in analyzing chromatograms; for use in controlling microfluidics; for use in controlling single-dimension and multi-dimensional electrophoresis and visualizing and analyzing electrophoretic results; for use in protein analysis and spectral processing; for use in analyzing, managing, and visualizing nucleic acid and amino acid sequences
- GS0421: Medical and diagnostic services, namely, diagnosing, preventing, and predicting disease, identifying hereditary risk factors, diagnostic testing, prognostic testing, gene and protein testing, identifying physiological characteristics, establishing individual preventative programs, establishing disease treatment based on gene and protein expression and/or activity, evaluating clinical trial progress, drug monitoring and computer tracking of a patient's health progress; research services, namely, research, development, validation, testing, data analysis and product development services in the biomedical, genomic, pharmacogenomic, diagnostic, clinical trial design, and biotechnology fields; research consulting services, namely, research, development, validation, testing, data analysis, and product development services for others in the biomedical, genomic, pharmaceutical, pharmacogenomic, diagnostic, clinical trial design, and biotechnology field; computer services, namely, providing databases featuring life sciences information via global, national, and local computer networks in the fields of pharmacogenomics, diagnostics, clinical trial design and biotechnology; computer consulting services, namely, providing databases featuring life sciences information to others via global, national, and local computer networks in the fields of pharmacogenomics, diagnostics, clinical trial design and biotechnology
Case File Event Statements
-
7/8/2011 - 13 years ago
21 - CANCELLED SEC. 8 (6-YR)
Type: C8..
-
1/13/2010 - 14 years ago
20 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
12/7/2004 - 19 years ago
19 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
5/11/2004 - 20 years ago
18 - PAPER RECEIVED
Type: MAIL
-
9/14/2004 - 19 years ago
17 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
8/25/2004 - 19 years ago
16 - NOTICE OF PUBLICATION
Type: NPUB
-
4/13/2004 - 20 years ago
15 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
3/2/2004 - 20 years ago
14 - Sec. 1(B) CLAIM DELETED
Type: 1.BD
-
3/2/2004 - 20 years ago
13 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
3/23/2004 - 20 years ago
12 - CASE FILE IN TICRS
Type: CFIT
-
3/23/2004 - 20 years ago
11 - CASE FILE IN TICRS
Type: CFIT
-
3/2/2004 - 20 years ago
10 - PAPER RECEIVED
Type: MAIL
-
9/2/2003 - 20 years ago
9 - INQUIRY AS TO SUSPENSION MAILED
Type: CNSI
-
2/24/2003 - 21 years ago
8 - LETTER OF SUSPENSION MAILED
Type: CNSL
-
1/24/2003 - 21 years ago
7 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
1/24/2003 - 21 years ago
6 - PAPER RECEIVED
Type: MAIL
-
10/31/2002 - 21 years ago
5 - INQUIRY AS TO SUSPENSION MAILED
Type: CNSI
-
4/5/2002 - 22 years ago
4 - LETTER OF SUSPENSION MAILED
Type: CNSL
-
2/8/2002 - 22 years ago
3 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
8/10/2001 - 22 years ago
2 - NON-FINAL ACTION MAILED
Type: CNRT
-
8/3/2001 - 22 years ago
1 - ASSIGNED TO EXAMINER
Type: DOCK