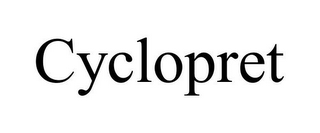Information
-
 Trademark
Trademark
-
 77220721
77220721
-
Serial Number
77220721
-
Registration Number
3623627
-
International Classifications
-
Filing Date
July 02, 2007
18 years ago
-
Registration Date
May 19, 2009
16 years ago
-
Transaction Date
December 26, 2015
9 years ago
-
Status Date
December 25, 2015
9 years ago
-
Published for Opposition Date
December 11, 2007
17 years ago
-
Location Date
April 13, 2009
16 years ago
-
First Use Anywhere Date
November 01, 2007
18 years ago
-
First Use In Commerce Date
November 01, 2007
18 years ago
-
Status Code
710
-
Current Location
PUBLICATION AND ISSUE SECTION
Employee Name
CAPSHAW, DANIEL
-
Attorney Docket Number
1070-TM-144
Attorney Name
Kenneth F. Florek
Law Office Assigned Location Code
M10
-
Owners
Mark Drawing Code
4000
Mark Identification
CYCLOPRET
Case File Statements
- GS0051: Pharmaceutical and veterinary preparations for the treatment of gynecological disorders, namely, gynecological disorders affecting menstruation, menstrual irregularities, polycystic ovarian syndrome, uterine bleeding, endometriosis and psychic complaints, nervousness, depression, mood disorders, mood changes, anxiety disorders, affective and cognitive disorders, hot flashes, perspiration, weeping spasm, headache, vertigo, palpitation, insomnia, premenstrual syndrome, menstrual disorders, mastodynia, mastalgia, mastopathy, gastrointestinal disorders, bone and joint pains, backache, breast pain, dizziness, headache, nausea, vomiting, diarrhea, tenseness, sexual dysfunction, acne, rashes, hirsutism, obesity, fertility disorders, hyperprolactinemia; pharmaceutical and veterinary preparations, namely, plant extracts for the treatment of gynecological disorders, namely, gynecological disorders affecting menstruation, menstrual irregularities, polycystic ovarian syndrome, uterine bleeding, endometriosis and psychic complaints, nervousness, depression, mood disorders, mood changes, anxiety disorders, affective and cognitive disorders, hot flashes, perspiration, weeping spasm, headache, vertigo, palpitation, insomnia, premenstrual syndrome, menstrual disorders, mastodynia, mastalgia, mastopathy, gastrointestinal disorders, bone and joint pains, backache, breast pain, dizziness, headache, nausea, vomiting, diarrhea, tenseness, sexual dysfunction, acne, rashes, hirsutism, obesity, fertility disorders, hyperprolactinemia; gynecologic oriental medicine for the treatment of gynecological disorders, namely, gynecological disorders affecting menstruation, menstrual irregularities, polycystic ovarian syndrome, uterine bleeding, endometriosis and psychic complaints, nervousness, depression, mood disorders, mood changes, anxiety disorders, affective and cognitive disorders, hot flashes, perspiration, weeping spasm, headache, vertigo, palpitation, insomnia, premenstrual syndrome, menstrual disorders, mastodynia, mastalgia, mastopathy, gastrointestinal disorders, bone and joint pains, backache, breast pain, dizziness, headache, nausea, vomiting, diarrhea, tenseness, sexual dysfunction, acne, rashes, hirsutism, obesity, fertility disorders, hyperprolactinemia; dietary supplements in the form of tablets, drops, capsules, extract preparations, balms and/or in liquid form, containing plant extracts for the treatment of gynecological disorders, namely, gynecological disorders affecting menstruation, menstrual irregularities, polycystic ovarian syndrome, uterine bleeding, endometriosis and psychic complaints, nervousness, depression, mood disorders, mood changes, anxiety disorders, affective and cognitive disorders, hot flashes, perspiration, weeping spasm, headache, vertigo, palpitation, insomnia, premenstrual syndrome, menstrual disorders, mastodynia, mastalgia, mastopathy, gastrointestinal disorders, bone and joint pains, backache, breast pain, dizziness, headache, nausea, vomiting, diarrhea, tenseness, sexual dysfunction, acne, rashes, hirsutism, obesity, fertility disorders, hyperprolactinemia; food supplements, dietary food supplements and/or herbal supplements in the form of tablets, drops, capsules, extract preparations, balms and/or in liquid form, containing plant extracts for the treatment of gynecological disorders, namely, gynecological disorders affecting menstruation, menstrual irregularities, polycystic ovarian syndrome, uterine bleeding, endometriosis and psychic complaints, nervousness, depression, mood disorders, mood changes, anxiety disorders, affective and cognitive disorders, hot flashes, perspiration, weeping spasm, headache, vertigo, palpitation, insomnia, premenstrual syndrome, menstrual disorders, mastodynia, mastalgia, mastopathy, gastrointestinal disorders, bone and joint pains, backache, breast pain, dizziness, headache, nausea, vomiting, diarrhea, tenseness, sexual dysfunction, acne, rashes, hirsutism, obesity, fertility disorders, hyperprolactinemia
Case File Event Statements
-
12/25/2015 - 9 years ago
21 - CANCELLED SEC. 8 (6-YR)
Type: C8..
-
4/27/2010 - 15 years ago
20 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
5/19/2009 - 16 years ago
19 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
4/13/2009 - 16 years ago
18 - LAW OFFICE REGISTRATION REVIEW COMPLETED
Type: REGV
-
4/11/2009 - 16 years ago
17 - ASSIGNED TO LIE
Type: ALIE
-
4/9/2009 - 16 years ago
16 - ALLOWED PRINCIPAL REGISTER - SOU ACCEPTED
Type: CNPR
-
3/16/2009 - 16 years ago
15 - STATEMENT OF USE PROCESSING COMPLETE
Type: SUPC
-
2/26/2009 - 16 years ago
14 - USE AMENDMENT FILED
Type: IUAF
-
3/16/2009 - 16 years ago
13 - CASE ASSIGNED TO INTENT TO USE PARALEGAL
Type: AITU
-
2/26/2009 - 16 years ago
12 - TEAS STATEMENT OF USE RECEIVED
Type: EISU
-
8/19/2008 - 17 years ago
11 - EXTENSION 1 GRANTED
Type: EX1G
-
8/19/2008 - 17 years ago
10 - EXTENSION 1 FILED
Type: EXT1
-
8/19/2008 - 17 years ago
9 - TEAS EXTENSION RECEIVED
Type: EEXT
-
3/4/2008 - 17 years ago
8 - NOA MAILED - SOU REQUIRED FROM APPLICANT
Type: NOAM
-
12/11/2007 - 17 years ago
7 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
11/21/2007 - 18 years ago
6 - NOTICE OF PUBLICATION
Type: NPUB
-
11/3/2007 - 18 years ago
5 - LAW OFFICE PUBLICATION REVIEW COMPLETED
Type: PREV
-
11/3/2007 - 18 years ago
4 - ASSIGNED TO LIE
Type: ALIE
-
10/1/2007 - 18 years ago
3 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
9/25/2007 - 18 years ago
2 - ASSIGNED TO EXAMINER
Type: DOCK
-
7/5/2007 - 18 years ago
1 - NEW APPLICATION ENTERED IN TRAM
Type: NWAP