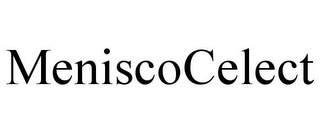Information
-
 Trademark
Trademark
-
 77238363
77238363
-
Serial Number
77238363
-
Registration Number
3662384
-
International Classifications
-
Filing Date
July 25, 2007
17 years ago
-
Registration Date
August 04, 2009
15 years ago
-
Transaction Date
January 24, 2014
11 years ago
-
Status Date
August 04, 2009
15 years ago
-
Published for Opposition Date
May 19, 2009
15 years ago
-
Location Date
August 04, 2009
15 years ago
-
Status Code
700
-
Current Location
PUBLICATION AND ISSUE SECTION
Employee Name
EULIN, INGRID C
-
Attorney Docket Number
MENI6001/TJM
Attorney Name
Thomas J. Moore
Law Office Assigned Location Code
M20
-
Owners
Mark Drawing Code
5000
Mark Identification
MENISCOCELECT
Case File Statements
- CC0000: Color is not claimed as a feature of the mark.
- GS0011: Enzymes for scientific research and development in the medical field; antibodies for medical laboratory use, for the purpose of expansion, selection, quality control and implantation of human, in vivo, cartilage forming cells including stem cell preparations of stem cell implants; quality control kits consisting primarily of monoclonal antibodies for use in disease testing and ligands or oligonucleotide primers and DNA chips for scientific or research use; quality control kits consisting primarily of antibodies or oligonucleotide primers and DNA chips for selection and quality control of human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants for research/scientific use
- GS0051: Antibodies for medical diagnostic use, for the purpose of expansion, selection, quality control and implantation of human, in vivo, cartilage forming cells including stem cell preparations of stem cell implants; pharmaceutical, veterinary or medical preparations for expansion, selection, quality control and implantation of human, in vivo, cartilage forming cells; nutritious media and growth substrates for expansion, selection, quality control and implantation of human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants; medical preparations, namely, polymer preparations and cell matrices of any type of origin for expansion, selection, medical quality control and implantation of human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants; test products for medical use, for the purpose of the control of expansion, selection, and implantation of human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants, namely, chondrocytes, chondrocyte progenitors, chondrocyte precursors and stem cells with chondrogenic properties; hormones for the promoting of the growth of cells called growth factors for medical use; veterinary or medical kits for expansion, selection, quality control and implantation of human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants, namely, kits primarily composed of growth and differentiating promoting polypeptides; quality control kits consisting primarily of monoclonal antibodies for use in disease testing and ligands or oligonucleotide primers and DNA chips for medical or veterinary use; quality control kits consisting primarily of antibodies or oligonucleotide primers and DNA chips for selection and quality control of human, in vivo, cartilage forming cells including stem cell preparations and stem cell implants for medical/diagnostic use; implementation kits consisting primarily of live cartilage forming cells
- GS0101: Veterinary or medical devices and instruments for expansion, selection, quality control and implantation of human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants, namely, kits comprised of temperature probes and injectors, stitching or gluing material for covering implantation devices for human in vivo, cartilage forming cells; medical devices and instrument kits, namely, biopsy instruments, implantation kits including medical syringes, tongs and pliers; quality control kits consisting primarily of DNA chips for medical use including expansion, selection, quality control and implantation of human, in vivo, cartilage forming cells including stem cell preparations and stem cell implants
- GS0421: Scientific research, development and study related to expansion, selection, quality control and implantation of human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants; quality control for others as related in human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants; custom design, development and performance of biochemical assays for the purpose of quality control of expanded or implanted human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants; scientific research, development and study related to the use of polymer membranes or cell matrices of any type of origin for expansion, selection, quality control and implantation of human, in vivo, cartilage forming cells including stem cell preparations or stem cell implants
- GS0441: Pharmaceutical, veterinary or medical services, namely, medical and pharmaceutical consultation and veterinary services
Case File Event Statements
-
1/23/2014 - 11 years ago
38 - APPLICANT/CORRESPONDENCE CHANGES (NON-RESPONSIVE) ENTERED
Type: CHAN
-
1/23/2014 - 11 years ago
37 - TEAS CHANGE OF OWNER ADDRESS RECEIVED
Type: COAR
-
8/4/2009 - 15 years ago
36 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
5/19/2009 - 15 years ago
35 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
4/29/2009 - 15 years ago
34 - NOTICE OF PUBLICATION
Type: NPUB
-
4/13/2009 - 15 years ago
33 - LAW OFFICE PUBLICATION REVIEW COMPLETED
Type: PREV
-
4/9/2009 - 15 years ago
32 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
4/9/2009 - 15 years ago
31 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
4/9/2009 - 15 years ago
30 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
4/9/2009 - 15 years ago
29 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
3/5/2009 - 15 years ago
28 - NOTIFICATION OF PRIORITY ACTION E-MAILED
Type: GPRN
-
3/5/2009 - 15 years ago
27 - PRIORITY ACTION E-MAILED
Type: GPRA
-
3/5/2009 - 15 years ago
26 - PRIORITY ACTION WRITTEN
Type: CPRA
-
2/9/2009 - 15 years ago
25 - PREVIOUS ALLOWANCE COUNT WITHDRAWN
Type: ZZZX
-
1/16/2009 - 16 years ago
24 - WITHDRAWN FROM PUB - OG REVIEW QUERY
Type: PBCR
-
1/8/2009 - 16 years ago
23 - LAW OFFICE PUBLICATION REVIEW COMPLETED
Type: PREV
-
12/20/2008 - 16 years ago
22 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
12/19/2008 - 16 years ago
21 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
12/19/2008 - 16 years ago
20 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
12/19/2008 - 16 years ago
19 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
6/21/2008 - 16 years ago
18 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
6/21/2008 - 16 years ago
17 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
6/21/2008 - 16 years ago
16 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
6/13/2008 - 16 years ago
15 - PREVIOUS ALLOWANCE COUNT WITHDRAWN
Type: ZZZX
-
6/2/2008 - 16 years ago
14 - WITHDRAWN FROM PUB - OG REVIEW QUERY
Type: PBCR
-
5/22/2008 - 16 years ago
13 - LAW OFFICE PUBLICATION REVIEW COMPLETED
Type: PREV
-
5/22/2008 - 16 years ago
12 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
5/1/2008 - 16 years ago
11 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
5/1/2008 - 16 years ago
10 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
4/30/2008 - 16 years ago
9 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
10/31/2007 - 17 years ago
8 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
10/31/2007 - 17 years ago
7 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
10/31/2007 - 17 years ago
6 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
10/30/2007 - 17 years ago
5 - ASSIGNED TO EXAMINER
Type: DOCK
-
10/25/2007 - 17 years ago
4 - APPLICANT AMENDMENT PRIOR TO EXAMINATION - ENTERED
Type: AMPX
-
10/18/2007 - 17 years ago
3 - ASSIGNED TO LIE
Type: ALIE
-
9/13/2007 - 17 years ago
2 - PAPER RECEIVED
Type: MAIL
-
7/30/2007 - 17 years ago
1 - NEW APPLICATION ENTERED IN TRAM
Type: NWAP