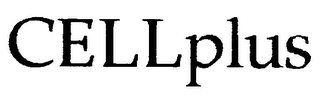Information
-
 Trademark
Trademark
-
 78299676
78299676
-
International Classifications
-
Filing Date
September 12, 2003
21 years ago
-
Transaction Date
July 23, 2011
13 years ago
-
Status Date
June 03, 2006
18 years ago
-
Location Date
June 03, 2006
18 years ago
-
Status Code
602
-
Current Location
TMO LAW OFFICE 114 - EXAMINING ATTORNEY ASSIGNED
Employee Name
NEVILLE, JAMES B
-
Attorney Docket Number
TM-1746
Attorney Name
C. Bruce Hamburg
Law Office Assigned Location Code
M50
-
Owners
Mark Drawing Code
5000
Mark Identification
CELLPLUS
Case File Statements
- GS0051: Biological implant materials and substitute materials for tissues of human or animal bodies consisting of living tissue being implant materials and substitute material for human bodies, living tissue being implant materials and substitute material for animal bodies; biological bone implant materials consisting of biological preparations for bone implant; replacement materials for human or animal body tissue made of bioceramics, plastics, maritime algae and/or metal; living tissue being replacement materials for human body, biological bone implant materials, replacement materials for human body tissue made of bioceramics, plastics, maritime algae and/or metal and replacement materials for animal body tissue made of bioceramics, plastics, maritime algae and/or metal
- GS0101: Artificial implants for use in human medicine, parts thereof dental-supraconstructures, namely dental crowns, dental bridges, dental prostheses, parts thereof apparatus, instruments and tools for surgical and dental purposes and for use in dental laboratories namely surgical apparatus for bone surgery; surgical apparatus for head surgery; medical instruments and tools for bone implants and prostheses operation; drills being parts of medical instruments and tools for bone implants and prostheses operation; cylinders being parts of medical instruments and tools for bone implants and prostheses operation; screw implant drivers being parts of medical; instruments and tools for bone implants and prostheses operation; hex drivers being parts of medical instruments and tools for bone implants and prostheses operation; bone condensers being parts of medical instruments and tools for bone implants and prostheses operation; bone expanders being parts of medical instruments and tools for bone implants and prostheses operation; handles being parts of medical instruments and tools for bone implants and prostheses operation; periotome blades being parts of medical instruments and tools for bone implants and prostheses operation; pins being parts of medical instruments and tools for bone implants and prostheses operation; ratchets being parts of medical instruments and tools for bone implants and prostheses operation; gauges being parts of medical instruments and tools for bone implants and prostheses operation; implant mallets being parts of medical instruments and tools for bone implants and prostheses operation; implant forceps being parts of medical instruments and tools for bone implants and prostheses operation; tissue punches being parts of medical instruments and tools for bone implants and prostheses operation; bone profilers being parts of medical instruments and tools for bone implants and prostheses operation; activators and deactivators for ball and socket attachment being parts of medical instruments and tools for bone implants and prostheses operation; bar clip activators being parts of medical instruments and tools for bone implants and prostheses operation; finishers for abutment seat and screw head seat being parts of medical instruments and tools for bone implants and prostheses operation; membranes for covering bone defects and pins for fixing these membranes on the bone; parts for the aforesaid goods; cases, bags and boxes for medical and dental purposes and for use in dental laboratories, especially for the storage and for the sterilization of the aforesaid apparatus, instruments, tools, implants and suprastructures; furniture for medical and dental use and for use in dental laboratories; membranes for covering bone defects as well as pins for fixing these membranes on the bone; bone implant materials made of titanium, implant materials made of titanium substituting the root of the tooth, implants for osteotomy and parts thereof
- PM0000: CELL PLUS
Case File Event Statements
-
6/5/2006 - 18 years ago
15 - ABANDONMENT NOTICE MAILED - FAILURE TO RESPOND
Type: MAB2
-
6/3/2006 - 18 years ago
14 - ABANDONMENT - FAILURE TO RESPOND OR LATE RESPONSE
Type: ABN2
-
10/26/2005 - 19 years ago
13 - CORRESPONDENCE E-MAILED
Type: GRML
-
10/26/2005 - 19 years ago
12 - SUSPENSION INQUIRY WRITTEN
Type: CNSI
-
7/14/2005 - 19 years ago
11 - LIE CHECKED SUSP - TO ATTY FOR ACTION
Type: RCCK
-
12/7/2004 - 20 years ago
10 - LETTER OF SUSPENSION E-MAILED
Type: GNSL
-
12/7/2004 - 20 years ago
9 - SUSPENSION LETTER WRITTEN
Type: CNSL
-
11/12/2004 - 20 years ago
8 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
11/10/2004 - 20 years ago
7 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
11/10/2004 - 20 years ago
6 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
10/12/2004 - 20 years ago
5 - AMENDMENT FROM APPLICANT ENTERED
Type: ACEC
-
9/20/2004 - 20 years ago
4 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
9/23/2004 - 20 years ago
3 - PAPER RECEIVED
Type: MAIL
-
3/18/2004 - 20 years ago
2 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
3/12/2004 - 20 years ago
1 - ASSIGNED TO EXAMINER
Type: DOCK