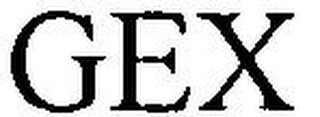Information
-
 Trademark
Trademark
-
 79068397
79068397
-
Serial Number
79068397
-
Registration Number
3870245
-
International Classifications
-
Filing Date
November 27, 2008
16 years ago
-
Registration Date
November 02, 2010
14 years ago
-
Transaction Date
October 02, 2022
2 years ago
-
Status Date
October 30, 2021
3 years ago
-
Published for Opposition Date
August 17, 2010
14 years ago
-
Location Date
October 30, 2021
3 years ago
-
Status Code
707
-
Employee Name
MORRIS, KRISTINA KLOIB
-
Attorney Docket Number
S14321
Attorney Name
Leigh Ann Lindquist
Law Office Assigned Location Code
M70
-
Owners
Mark Drawing Code
4000
Mark Identification
GEX
Case File Statements
- A00002: In the statement, Page 2, line 17 through line 22, "sanitary preparations for medical purposes; dietetic foods adapted for medical use; food for babies; medical plasters; materials for medical dressings; materials for dental filling and impression materials for dental purposes; disinfectants for sanitary purposes; preparations for destroying vermin; fungicides, herbicides; adjuvants for medical purposes; " is deleted. In the statement Page 2, line 23, "and veterinary" is deleted. In the statement, Page 2, line 25, "allergies, cardiovascular diseases," is deleted. In the statement, Page 2, line 25 through line 26, "neurological diseases" is deleted.
- A00001: In the statement, Page 1, line 35, through 39 is deleted. In the statement, Page 2, line 1, "microorganism and" is deleted. In the statement, Page 2, line 4, "microorganism or" is deleted. In the statement, Page 3, line 27, "bacteria" is deleted. In the statement, Page 3, line 30, "bacteria" is deleted. In the statement, Page 3, line 37, "chemistry" is deleted. In the statement, Page 3, line 49 "and microorganisms" is deleted. In the statement, Page 3, lines 53-54, "microorganisms and" is deleted.
- A00003: In the statement, Page 2, line 13 through line 57 is deleted, and Page 3, line 1 through line 20 is deleted, and, medicines for human purposes for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine is inserted.
- GS0051: [ Pharmaceutical and veterinary preparations for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, allergies, cardiovascular diseases, infectious diseases, neurological diseases, gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine; sanitary preparations for medical purposes; dietetic foods adapted for medical use; food for babies; medical plasters; materials for medical dressings; materials for dental filling and impression materials for dental purposes; disinfectants for sanitary purposes; preparations for destroying vermin; fungicides, herbicides; adjuvants for medical purposes; Medicines for human and veterinary purposes for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, [ allergies, cardiovascular diseases, ] infectious diseases, [ neurological diseases, ] gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine; biological preparations for medical purposes; dietetic food adapted for medical purposes; enzyme, proteins, antibodies, cells, microorganisms as well as fragments, fractions, elements and lysates thereof for medical purposes for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, allergies, cardiovascular diseases, infectious diseases, neurological diseases, gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine, and as nutritional additives for medical purposes for use in foods and dietary supplements for human consumption; vaccines; carbohydrates for medical purposes for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, allergies, cardiovascular diseases, infectious diseases, neurological diseases, gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine; cultures of microorganisms for medical or veterinary purposes for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, allergies, cardiovascular diseases, infectious diseases, neurological diseases, gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine; cultures of human cells for medical or veterinary purposes for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, allergies, cardiovascular diseases, infectious diseases, neurological diseases, gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine; medical drinks for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, allergies, cardiovascular diseases, infectious diseases, neurological diseases, gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine; microorganisms, cells and preparations containing elements, fragments, fractions and lysates of microorganisms or cells for medical and veterinary purposes for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, allergies, cardiovascular diseases, infectious diseases, neurological diseases, gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine; biological therapeutics, namely, therapeutics containing cells, microorganisms, proteins, glycoproteins, peptides, glycopeptides or modified or conjugated versions thereof ; milk ferments for pharmaceutical purposes for prophylaxis or treatment of cancer, inflammatory diseases, autoimmune diseases, allergies, cardiovascular diseases, infectious diseases, neurological diseases, gastrointestinal diseases, and for use in wound healing, formation of blood and tissue and reproduction medicine; nutritional additives and nutritional supplement additives for medical purposes for use in foods and dietary supplements for human consumption; nutrients for medical purposes, namely, enzymes, proteins, antibodies, cells, microorganisms as well as fragments, fractions, elements and lysates thereof as nutritional additives; sugar and carbohydrates for medical purposes, namely, therapeutically and prophylactically active sugar and carbohydrates, dietetic sugar and carbohydrates, sugar and carbohydrate replacements ]
- GS0011: [ Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry, except fungicides, herbicides, insecticides and parasiticides; unprocessed artificial resins; unprocessed plastics; fertilising preparations; fire extinguishing compositions; tempering and soldering chemicals; chemical substances for fermenting and preserving food stuffs; tanning agents for use in the manufacture of leather; adhesives used in industry; bacteria and cell preparations and elements, fragments, fractions and lysates thereof, except for medical or veterinary purposes, namely, for use in protein production, food manufacturing, scientific research, waste water treatment, bacteria and cell preparations for use in expression of engineered or glycosylated proteins and peptides; preparations containing bacteria and cells, except for medical or veterinary purposes, namely, for use in protein production, food manufacturing, scientific research, waste water treatment; biological preparations for laboratories, except for medical or veterinary purposes, namely, for use in bacterial and cell engineering, cultivation, harvesting, protein production; chemical preparations for analysis in laboratories, namely, preparations for protein, peptide, engineered proteins and peptides, glycoproteins, glycosylation, antibody or antigen analysis, preparations comprising antibody reagents used for the detection of antigens; chemical preparations for scientific purposes; chemical reagents except for medical or veterinary purposes and biological reagents in the nature of reagents for use in protein production, bacterial and cell engineering, cultivation and harvesting, probes for detecting and analyzing molecules in protein or nucleotide arrays, except for medical or veterinary purposes; diagnostic preparations, except for medical or veterinary purposes; enzymes, proteins and respective preparations for industrial purposes, namely, engineered proteins, glycosylated proteins, antibodies, enzymes for use in glycosylation, enzyme substrates, proteins used in the manufacture of cosmetics, beverages, food products and food supplements; ferments for chemical purposes, namely, yeast, bacterium, mold, or enzyme, that causes fermentation; in-vitro diagnostics, other than for medical purposes; carbohydrates for use in the manufacture of food and nutraceuticals; cultures of microorganisms and cells and elements, fragments, fractions and lysates thereof, other than for medical or veterinary purposes, namely, cultures of microorganisms and cells other than for medical and veterinary use, microorganism or cell cultures for use in protein production, food manufacturing, scientific research, waste water treatment, cultures of microorganisms or cells for use in expression of glycosylated proteins and peptides; expression systems for proteins and peptides comprising cells, particularly human cells and microorganisms suitable for expressing engineered or glycosylated proteins or peptides, or capable of providing optimized glycosylation; milk ferments for medical research purposes ]
- GS0421: Scientific and technological services and research and designer services relating thereto, namely, scientific research, scientific analysis, technology consultation and research, product development and optimization in the field of biotechnology, protein production, glycosylation, optimization of protein, antibody and enzyme activity, [ bacteria ] and cell engineering, cultivation and harvesting; industrial analysis and research services in the field of biotechnology, protein production, glycosylation, protein, antibody and enzyme activity, [ bacteria ] and cell engineering, cultivation and harvesting; [ design and development of computer hardware and software; ] biological research; services of a [ chemical or ] biological laboratory; [ technical surveys, namely, ] conducting biotechnology surveys, [ pharmacology surveys; ] conducting scientific surveys in the field of biotechnology, [ bacterial and ] cell engineering, protein production, glycosylation; research and development in the field of [ bacteriology, ] cytology, antibody technology, [ chemistry, ] immunology, [ cosmetics of expression systems in the nature of glycosylation of proteins, peptides, antibodies and enzymes, particularly for proteins and peptides; quality control; ] research and development of new products and technologies in the field of biotechnology, protein production, glycosylation, protein, antibody and enzyme activity, [ bacteria and ] cell engineering, cultivation and harvesting for others; technology and scientific consultancy in the field of biotechnology, protein production, glycosylation, protein, antibody and enzyme activity, [ bacteria and ] cell engineering, cultivation and harvesting; research and development in the field of glycomics, expression systems in the nature of glycosylation of proteins, peptides, antibodies and enzymes, development and improvement of cells, cell lines [ and microorganisms, ] antibody-engineering, optimised biological therapeutics, [ nutraceuticals, and food and nutritional supplements; ] research, quality control, technology consultancy and product development in the field of proteins, antibodies, [ microorganisms ] and cells; research and development in the field of [ microorganisms and ] cells
Case File Event Statements
-
10/2/2022 - 2 years ago
60 - PARTIAL INVALIDATION PROCESSED BY THE IB
Type: INNP
-
8/23/2022 - 2 years ago
59 - PARTIAL INVALIDATION OF REG EXT PROTECTION SENT TO IB
Type: INPS
-
8/23/2022 - 2 years ago
58 - INVALIDATION PROCESSED
Type: INPC
-
6/30/2022 - 3 years ago
57 - PARTIAL INVALIDATION OF REG EXT PROTECTION CREATED
Type: INPR
-
10/30/2021 - 3 years ago
56 - NOTICE OF ACCEPTANCE OF SEC. 71 - E-MAILED
Type: NA71
-
10/30/2021 - 3 years ago
55 - REGISTERED - PARTIAL SEC 71 ACCEPTED
Type: 71.P
-
10/28/2021 - 3 years ago
54 - TEAS RESPONSE TO OFFICE ACTION-POST REG RECEIVED
Type: EROP
-
5/6/2021 - 4 years ago
53 - POST REGISTRATION ACTION MAILED - SEC.71
Type: PR71
-
1/20/2021 - 4 years ago
52 - CASE ASSIGNED TO POST REGISTRATION PARALEGAL
Type: APRE
-
11/2/2020 - 4 years ago
51 - TEAS SECTION 71 RECEIVED
Type: ES71
-
7/8/2020 - 5 years ago
50 - AMENDMENT UNDER SECTION 7 ¿ PROCESSED
Type: A7OK
-
12/16/2019 - 5 years ago
49 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
12/16/2019 - 5 years ago
48 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
11/2/2019 - 5 years ago
47 - COURTESY REMINDER - SEC. 71 (10-YR) E-MAILED
Type: REM4
-
12/13/2018 - 6 years ago
46 - INTERNATIONAL REGISTRATION RENEWED
Type: RNWL
-
10/4/2018 - 6 years ago
45 - CASE ASSIGNED TO POST REGISTRATION PARALEGAL
Type: APRE
-
6/1/2018 - 7 years ago
44 - TEAS SECTION 7 REQUEST RECEIVED
Type: ES7R
-
3/30/2018 - 7 years ago
43 - AMENDMENT UNDER SECTION 7 ¿ PROCESSED
Type: A7OK
-
11/30/2017 - 7 years ago
42 - CASE ASSIGNED TO POST REGISTRATION PARALEGAL
Type: APRE
-
10/20/2017 - 7 years ago
41 - TEAS SECTION 7 REQUEST RECEIVED
Type: ES7R
-
6/23/2017 - 8 years ago
40 - NOTICE OF ACCEPTANCE OF SEC. 71 & 15 - E-MAILED
Type: NA75
-
6/23/2017 - 8 years ago
39 - REGISTERED - SEC. 71 ACCEPTED & SEC. 15 ACK.
Type: C75A
-
6/20/2017 - 8 years ago
38 - CASE ASSIGNED TO POST REGISTRATION PARALEGAL
Type: APRE
-
4/27/2017 - 8 years ago
37 - TEAS SECTION 71 & 15 RECEIVED
Type: ES75
-
12/14/2013 - 11 years ago
36 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
2/18/2013 - 12 years ago
35 - FINAL DECISION TRANSACTION PROCESSED BY IB
Type: FINO
-
2/11/2011 - 14 years ago
34 - FINAL DISPOSITION NOTICE SENT TO IB
Type: FICS
-
2/11/2011 - 14 years ago
33 - FINAL DISPOSITION PROCESSED
Type: FIMP
-
2/2/2011 - 14 years ago
32 - FINAL DISPOSITION NOTICE CREATED, TO BE SENT TO IB
Type: FICR
-
11/2/2010 - 14 years ago
31 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
8/17/2010 - 14 years ago
30 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
8/17/2010 - 14 years ago
29 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
7/15/2010 - 15 years ago
28 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
7/16/2010 - 15 years ago
27 - EXAMINERS AMENDMENT MAILED
Type: CNEA
-
7/15/2010 - 15 years ago
26 - LAW OFFICE PUBLICATION REVIEW COMPLETED
Type: PREV
-
7/15/2010 - 15 years ago
25 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
7/15/2010 - 15 years ago
24 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
7/15/2010 - 15 years ago
23 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
7/15/2010 - 15 years ago
22 - PREVIOUS ALLOWANCE COUNT WITHDRAWN
Type: ZZZX
-
7/15/2010 - 15 years ago
21 - EXAMINERS AMENDMENT MAILED
Type: CNEA
-
7/15/2010 - 15 years ago
20 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
7/15/2010 - 15 years ago
19 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
7/15/2010 - 15 years ago
18 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
1/20/2010 - 15 years ago
17 - FINAL REFUSAL MAILED
Type: CNFR
-
1/19/2010 - 15 years ago
16 - FINAL REFUSAL WRITTEN
Type: CNFR
-
12/31/2009 - 15 years ago
15 - CORRECTION FROM THE IB EXAMINED, NO ACTION IS NEEDED
Type: CORN
-
1/1/2010 - 15 years ago
14 - CORRECTION TRANSACTION RECEIVED FROM IB
Type: CRCV
-
12/28/2009 - 15 years ago
13 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
12/28/2009 - 15 years ago
12 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
12/28/2009 - 15 years ago
11 - ASSIGNED TO LIE
Type: ALIE
-
12/14/2009 - 15 years ago
10 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
7/10/2009 - 16 years ago
9 - REFUSAL PROCESSED BY IB
Type: RFNT
-
6/15/2009 - 16 years ago
8 - NON-FINAL ACTION MAILED - REFUSAL SENT TO IB
Type: RFCS
-
6/15/2009 - 16 years ago
7 - REFUSAL PROCESSED BY MPU
Type: RFRR
-
6/13/2009 - 16 years ago
6 - NON-FINAL ACTION (IB REFUSAL) PREPARED FOR REVIEW
Type: RFCR
-
6/12/2009 - 16 years ago
5 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
6/9/2009 - 16 years ago
4 - APPLICATION FILING RECEIPT MAILED
Type: MAFR
-
6/5/2009 - 16 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
6/5/2009 - 16 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM
Type: NWOS
-
6/4/2009 - 16 years ago
1 - SN ASSIGNED FOR SECT 66A APPL FROM IB
Type: REPR