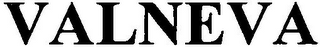Information
-
 Trademark
Trademark
-
 79138125
79138125
-
Serial Number
79138125
-
Registration Number
4686292
-
International Classifications
-
Filing Date
May 31, 2013
12 years ago
-
Registration Date
February 17, 2015
10 years ago
-
Transaction Date
May 10, 2025
8 months ago
-
Status Date
May 09, 2025
8 months ago
-
Published for Opposition Date
December 02, 2014
11 years ago
-
Location Date
May 09, 2025
8 months ago
-
Status Code
706
-
Current Location
Historical data usage
Employee Name
BUONGIORNO, CHRISTOPHER
-
Attorney Docket Number
I04222002200
Attorney Name
Christina M. Licursi
-
Owners
Mark Drawing Code
4
Mark Identification
VALNEVA
Case File Statements
- GS0051: Pharmaceuticals for human and veterinary use for the treatment of autoimmune diseases, inflammation, cancer, infectious diseases, diseases of the central nervous system, cardiovascular diseases, neurodegenerative and chronic diseases; antibodies and fragments thereof for use in disease testing; cell cultures, microorganisms and viruses for medical and veterinary use; diagnostic agents for medical, pharmaceutical and veterinary use; cells for medical or clinical use; culture media for cultivating microorganism cells for medical and veterinary use; adjuvants for medical or and veterinary use; pharmaceutical and veterinary preparations for medical, pharmaceutical and veterinary use, namely, preparations of encapsulated cells which release antibodies, living cells and microorganisms, vaccines, viral vectors, proteins, peptides, enzymes, nucleic acids or pharmaceutically active compounds for the treatment of cancer, auto-immune disorders, infectious diseases, inflammatory diseases, neurological and genetic disorders, bowel diseases, and digestion problems
- CC0000: Color is not claimed as a feature of the mark.
- GS0011: Chemicals for use in industry, science and agriculture, except fungicides, herbicides, insecticides and parasiticides; molecules from biotechnologies, namely, proteins in raw material for industrial use, scientific or medical research; cells and cell lines for use in scientific, laboratory, or medical research; nucleic acids for laboratory use; proteins in raw material form for scientific and medical research; recombinant proteins in raw material form for scientific and medical research; Biomedical compounds, namely, peptide substrates and peptide antigens used in analyzing and detecting certain toxins for clinical or medical laboratory use; Biochemical preparations derived from blood serum used as growth promoters in tissue cultures and microbiological media; Biochemicals, namely, antibodies and fragments thereof for in vitro scientific or research use; Chemicals for use in biotechnological product development; Cultures of microorganisms other than for medical and veterinary use; Biological preparations and bacteriological preparations for use in cell cultures other than for medical or veterinary use
- GS0421: Scientific research services for medical purposes in the fields of autoimmune diseases, inflammation, cancer, infectious diseases, diseases of the central nervous system, cardiovascular diseases, neurodegenerative and chronic diseases; scientific research for medical, pharmaceutical and veterinary purposes in the fields of autoimmune diseases, inflammation, cancer, infectious diseases, diseases of the central nervous system, cardiovascular diseases, neurodegenerative and chronic diseases; scientific research, in particular, in the fields of biology, cellular and molecular biology, biotechnology, bacteriology, immunology, genetics, medicine, pharmacology, virology, chemistry; scientific research relating to vaccines, antibiotics and immunological products for human and veterinary use; research and development of new products for others; testing, analysis, and evaluation of the goods of others to assure compliance with industry standards in the scientific, medical and veterinary fields; development and design of laboratory instruments for scientific and industrial research, in particular, to obtain and prepare cells, cell lines, viruses, proteins, animal and human materials; development of vaccines, antibiotics and immunological products for human and veterinary use; scientific and technological services, namely, diagnostic and prognostic analyses in the fields of cells, cell lines, animal and human materials, microorganisms, molecules for scientific purposes; scientific research, namely, preparation of scientific experts opinions reports
Case File Event Statements
-
11/20/2013 - 12 years ago
8 - NON-FINAL ACTION MAILED - REFUSAL SENT TO IB
Type: RFCS
-
11/11/2013 - 12 years ago
1 - SN ASSIGNED FOR SECT 66A APPL FROM IB
Type: REPR
-
11/13/2013 - 12 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
11/13/2013 - 12 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
11/15/2013 - 12 years ago
4 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
11/16/2013 - 12 years ago
5 - NON-FINAL ACTION (IB REFUSAL) PREPARED FOR REVIEW
Type: RFCR
-
11/19/2013 - 12 years ago
6 - APPLICATION FILING RECEIPT MAILED
Type: MAFR
-
11/20/2013 - 12 years ago
7 - REFUSAL PROCESSED BY MPU
Type: RFRR
-
10/9/2014 - 11 years ago
18 - NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED
Type: GNEN
-
12/10/2013 - 12 years ago
9 - REFUSAL PROCESSED BY IB
Type: RFNT
-
4/9/2014 - 11 years ago
10 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
4/9/2014 - 11 years ago
11 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
4/9/2014 - 11 years ago
12 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
4/24/2014 - 11 years ago
13 - FINAL REFUSAL WRITTEN
Type: CNFR
-
4/24/2014 - 11 years ago
14 - FINAL REFUSAL E-MAILED
Type: GNFR
-
4/24/2014 - 11 years ago
15 - NOTIFICATION OF FINAL REFUSAL EMAILED
Type: GNFN
-
10/9/2014 - 11 years ago
16 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
10/9/2014 - 11 years ago
17 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
10/9/2014 - 11 years ago
19 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
10/9/2014 - 11 years ago
20 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
6/29/2015 - 10 years ago
28 - FINAL DISPOSITION PROCESSED
Type: FIMP
-
10/28/2014 - 11 years ago
21 - ASSIGNED TO LIE
Type: ALIE
-
10/29/2014 - 11 years ago
22 - LAW OFFICE PUBLICATION REVIEW COMPLETED
Type: PREV
-
11/12/2014 - 11 years ago
23 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
12/2/2014 - 11 years ago
24 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
12/2/2014 - 11 years ago
25 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
2/17/2015 - 10 years ago
26 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
5/17/2015 - 10 years ago
27 - FINAL DISPOSITION NOTICE CREATED, TO BE SENT TO IB
Type: FICR
-
9/26/2019 - 6 years ago
33 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
6/29/2015 - 10 years ago
29 - FINAL DISPOSITION NOTICE SENT TO IB
Type: FICS
-
7/31/2015 - 10 years ago
30 - FINAL DECISION TRANSACTION PROCESSED BY IB
Type: FINO
-
12/22/2016 - 9 years ago
31 - CHANGE OF NAME/ADDRESS REC'D FROM IB
Type: ADCH
-
12/13/2018 - 7 years ago
32 - CHANGE OF NAME/ADDRESS REC'D FROM IB
Type: ADCH
-
2/17/2020 - 5 years ago
34 - COURTESY REMINDER - SEC. 71 (6-YR) E-MAILED
Type: REM3
-
7/8/2020 - 5 years ago
35 - TEAS SECTION 71 & 15 RECEIVED
Type: ES75
-
11/24/2020 - 5 years ago
36 - CASE ASSIGNED TO POST REGISTRATION PARALEGAL
Type: APRE
-
11/30/2020 - 5 years ago
37 - REGISTERED - SEC. 71 ACCEPTED & SEC. 15 ACK.
Type: C75A
-
11/30/2020 - 5 years ago
38 - NOTICE OF ACCEPTANCE OF SEC. 71 & 15 - E-MAILED
Type: NA75
-
10/1/2022 - 3 years ago
39 - RESTRICTION OF HOLDER'S RIGHT OF DISPOSAL RECEIVED
Type: RHRD
-
10/15/2022 - 3 years ago
40 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
11/3/2022 - 3 years ago
41 - LIMITATION FROM THE IB EXAMINED, NO ACTION IS NEEDED
Type: LIMN
-
5/22/2024 - a year ago
47 - LIMITATION FROM THE IB EXAMINED, NO ACTION IS NEEDED
Type: LIMN
-
4/7/2023 - 2 years ago
42 - INTERNATIONAL REGISTRATION RENEWED
Type: RNWL
-
2/17/2024 - a year ago
44 - RESTRICTION OF HOLDER'S RIGHT OF DISPOSAL RECEIVED
Type: RHRD
-
2/17/2024 - a year ago
43 - COURTESY REMINDER - SEC. 71 (10-YR) E-MAILED
Type: REM4
-
2/25/2024 - a year ago
45 - RESTRICTION OF HOLDER'S RIGHT OF DISPOSAL RECEIVED
Type: RHRD
-
4/25/2024 - a year ago
46 - LIMITATION FROM THE IB EXAMINED, NO ACTION IS NEEDED
Type: LIMN
-
12/27/2024 - a year ago
48 - TEAS SECTION 71 RECEIVED
Type: ES71
-
5/5/2025 - 9 months ago
49 - CASE ASSIGNED TO POST REGISTRATION PARALEGAL
Type: APRE
-
5/9/2025 - 8 months ago
50 - REGISTERED-SEC.71 ACCEPTED
Type: 71AG
-
5/9/2025 - 8 months ago
51 - NOTICE OF ACCEPTANCE OF SEC. 71 - E-MAILED
Type: NA71