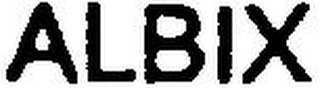Information
-
 Trademark
Trademark
-
 79141004
79141004
-
Serial Number
79141004
-
Registration Number
4607782
-
International Classifications
-
Filing Date
October 09, 2013
12 years ago
-
Registration Date
September 23, 2014
11 years ago
-
Transaction Date
May 17, 2024
a year ago
-
Status Date
October 08, 2023
2 years ago
-
Published for Opposition Date
July 08, 2014
11 years ago
-
Location Date
February 28, 2016
9 years ago
-
Status Code
404
-
Current Location
POST REGISTRATION
Employee Name
MAKHDOOM, SAIMA
-
Attorney Docket Number
10249-134
Law Office Assigned Location Code
L10
-
Owners
Mark Drawing Code
4
Mark Identification
ALBIX
Case File Statements
- B00001: Line 33, "in the field of development of medicines and pharmaceutical products" should be inserted after "cells" in class 42.
- GS0051: Pharmaceutical products and substances; reagents * for pharmaceutical and medical use * ; diagnostic reagents * for medical use * ; proteins * for pharmaceutical and medical use * ; albumin * for pharmaceutical and medical use * ; recombinant albumin * for pharmaceutical and medical use * ; diagnostic imaging agents
- B00002: In the Statement, Page 1, lines 5 - 11, "Chemicals used in industry, science, agriculture, horticulture and forestry except herbicides, fungicides, parasiticides and insecticides; chemicals for use in medicines and in the preparation of pharmaceuticals, namely, excipients for the stabilisation of pharmaceuticals and vaccines; reagents, namely, medium ingredient for the growth of animal cell lines; diagnostic reagents for biochemical analysis; proteins, namely, proteins for industrial use; " should be deleted, and, In the Statement, Page 1, lines 15-17, "; chemical substances for use in tissue culture, namely, cell growth media for growing animal cells, stem cells and cell therapy preparations" should be deleted, and, In the Statement, Page 1, line 19, after reagents, "for pharmaceutical and medical use" should be inserted, and, In the Statement, Page 1, line 20 after REAGENTS, "for medical use" should be inserted, and, In the Statement, Page 1, line 20, after PROTEINS, "for pharmaceutical and medical use" should be inserted, and, In the Statement, Page 1, line 20, after ALBUMIN, "for pharmaceutical and medical use" should be inserted, and, In the Statement, Page 1, line 20, after, RECOMBINANT ALBUMIN, "for pharmaceutical and medical use" should be inserted, and, In the Statement, Page 2, line 1, after ALL RELATING TO, "the use of albumin in" should be inserted.
- GS0011: [ Chemicals used in industry, science, agriculture, horticulture and forestry except herbicides, fungicides, parasiticides and insecticides; chemicals for use in medicines and in the preparation of pharmaceuticals, namely, excipients for the stabilisation of pharmaceuticals and vaccines; reagents, namely, medium ingredient for the growth of animal cell lines; diagnostic reagents for biochemical analysis; proteins, namely, proteins for industrial use; ] albumin for use in the manufacture of pharmaceuticals, vaccines, medical substances and cell therapy preparations; chemical substances for use in diagnostic kits, namely, albumin blocking agents used to bind to test kit surfaces to prevent non-specific binding of test substances to the surface [ ; chemical substances for use in tissue culture, namely, cell growth media for growing animal cells, stem cells and cell therapy preparations ]
- GS0421: Scientific and technological research and development services for new products and uses in the fields of biopharmaceuticals, pharmaceuticals, vaccines, medical devices, cell therapies and stem cells; consultancy services regarding product research and product development in the fields of biopharmaceuticals, pharmaceuticals, vaccines, medical devices, cell therapies, and stem cell research; advisory services with respect to research, development and suitable uses of biopharmaceuticals, pharmaceuticals, vaccines, medical devices, cell therapies and stem cells; providing expert analysis with respect to research, development and suitable uses of biopharmaceuticals, pharmaceuticals, vaccines, medical devices, cell therapies, and stem cells * in the field of development of medicines and pharmaceutical products *; and scientific development and analysis in the fields of pharmaceuticals, vaccines, and drug discoveries; all relating to * the use of albumin in * science, biotechnology, biochemistry, biology, medicine and pharmaceuticals
Case File Event Statements
-
1/2/2014 - 11 years ago
1 - SN ASSIGNED FOR SECT 66A APPL FROM IB
Type: REPR
-
1/8/2014 - 11 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
1/14/2014 - 11 years ago
3 - APPLICATION FILING RECEIPT MAILED
Type: MAFR
-
1/25/2014 - 11 years ago
4 - ASSIGNED TO EXAMINER
Type: DOCK
-
2/1/2014 - 11 years ago
6 - NON-FINAL ACTION (IB REFUSAL) PREPARED FOR REVIEW
Type: RFCR
-
2/3/2014 - 11 years ago
8 - NON-FINAL ACTION MAILED - REFUSAL SENT TO IB
Type: RFCS
-
2/22/2014 - 11 years ago
11 - REFUSAL PROCESSED BY IB
Type: RFNT
-
5/26/2014 - 11 years ago
12 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
1/31/2014 - 11 years ago
5 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
2/3/2014 - 11 years ago
7 - REFUSAL PROCESSED BY MPU
Type: RFRR
-
2/19/2014 - 11 years ago
9 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
2/19/2014 - 11 years ago
10 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
5/26/2014 - 11 years ago
13 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
5/26/2014 - 11 years ago
14 - NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED
Type: GNEN
-
6/5/2014 - 11 years ago
18 - LAW OFFICE PUBLICATION REVIEW COMPLETED
Type: PREV
-
6/18/2014 - 11 years ago
19 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
7/2/2014 - 11 years ago
21 - NOTIFICATION OF POSSIBLE OPPOSITION SENT TO IB
Type: OPNS
-
5/27/2014 - 11 years ago
15 - ASSIGNED TO LIE
Type: ALIE
-
5/27/2014 - 11 years ago
16 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
5/27/2014 - 11 years ago
17 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
7/2/2014 - 11 years ago
20 - NOTICE OF START OF OPPOSITION PERIOD CREATED, TO BE SENT TO IB
Type: OP2R
-
7/8/2014 - 11 years ago
22 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
7/8/2014 - 11 years ago
23 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
9/23/2014 - 11 years ago
25 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
12/23/2014 - 10 years ago
30 - FINAL DISPOSITION NOTICE CREATED, TO BE SENT TO IB
Type: FICR
-
2/27/2015 - 10 years ago
31 - FINAL DISPOSITION PROCESSED
Type: FIMP
-
7/22/2014 - 11 years ago
24 - NOTIFICATION PROCESSED BY IB
Type: GPNX
-
10/30/2014 - 11 years ago
26 - LIMITATION OF GOODS RECEIVED FROM IB
Type: LIMG
-
11/19/2014 - 10 years ago
27 - LIMITATION FROM THE IB EXAMINED AND ENTERED
Type: LIME
-
11/19/2014 - 10 years ago
28 - ASSIGNED TO PARALEGAL
Type: PLGL
-
11/19/2014 - 10 years ago
29 - CORRECTION UNDER SECTION 7 - PROCESSED
Type: COC.
-
2/28/2016 - 9 years ago
41 - CORRECTION UNDER SECTION 7 - PROCESSED
Type: COC.
-
2/27/2015 - 10 years ago
32 - FINAL DISPOSITION NOTICE SENT TO IB
Type: FICS
-
3/20/2015 - 10 years ago
33 - FINAL DECISION TRANSACTION PROCESSED BY IB
Type: FINO
-
7/20/2015 - 10 years ago
34 - PARTIAL INVALIDATION OF REG EXT PROTECTION CREATED
Type: INPR
-
8/20/2015 - 10 years ago
35 - INVALIDATION REVIEWED - NO ACTION REQUIRED BY OFFICE
Type: INNA
-
10/22/2015 - 10 years ago
36 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
10/22/2015 - 10 years ago
37 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
2/19/2016 - 9 years ago
38 - LIMITATION OF GOODS RECEIVED FROM IB
Type: LIMG
-
2/24/2016 - 9 years ago
39 - LIMITATION FROM THE IB EXAMINED AND ENTERED
Type: LIME
-
2/26/2016 - 9 years ago
40 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
10/28/2016 - 9 years ago
43 - PARTIAL INVALIDATION OF REG EXT PROTECTION CREATED
Type: INPR
-
9/23/2019 - 6 years ago
50 - COURTESY REMINDER - SEC. 71 (6-YR) E-MAILED
Type: REM3
-
4/27/2016 - 9 years ago
42 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
11/9/2016 - 9 years ago
44 - INVALIDATION REVIEWED - NO ACTION REQUIRED BY OFFICE
Type: INNA
-
7/13/2017 - 8 years ago
45 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
2/1/2018 - 7 years ago
46 - CHANGE OF NAME/ADDRESS REC'D FROM IB
Type: ADCH
-
10/11/2018 - 7 years ago
47 - CHANGE OF OWNER RECEIVED FROM IB
Type: CHLD
-
1/24/2019 - 6 years ago
48 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
3/14/2019 - 6 years ago
49 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
1/23/2020 - 5 years ago
51 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
4/9/2021 - 4 years ago
52 - CANCELLED SECTION 71
Type: C71T
-
7/4/2022 - 3 years ago
57 - TOTAL INVALIDATION PROCESSED BY THE IB
Type: INNT
-
4/23/2021 - 4 years ago
53 - CHANGE OF NAME/ADDRESS REC'D FROM IB
Type: ADCH
-
12/9/2021 - 3 years ago
54 - TOTAL INVALIDATION OF REG EXT PROTECTION CREATED
Type: INTR
-
4/22/2022 - 3 years ago
55 - INVALIDATION PROCESSED
Type: INPC
-
4/22/2022 - 3 years ago
56 - TOTAL INVALIDATION OF REG EXT PROTECTION SENT TO IB
Type: INTS
-
1/29/2023 - 2 years ago
58 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
5/6/2024 - a year ago
60 - DEATH OF INTERNATIONAL REGISTRATION
Type: DETH
-
5/6/2024 - a year ago
61 - NOTIFICATION OF EFFECT OF CANCELLATION OF INTL REG E-MAILED
Type: DENA
-
5/6/2024 - a year ago
62 - NOTIFICATION OF EFFECT OF CANCELLATION OF INTL REG E-MAILED
Type: DENC
-
4/2/2023 - 2 years ago
59 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP