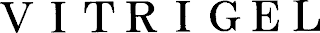Information
-
 Trademark
Trademark
-
 79274679
79274679
-
Serial Number
79274679
-
Registration Number
6138690
-
International Classifications
- 1 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry
- 5 - Pharmaceutical and veterinary preparations
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
- 10 - Surgical, medical, dental and veterinary apparatus and instruments, artificial limbs, eyes and teeth
-
Filing Date
July 25, 2019
5 years ago
-
Registration Date
September 01, 2020
4 years ago
-
Transaction Date
October 07, 2023
a year ago
-
Status Date
September 01, 2020
4 years ago
-
Published for Opposition Date
June 16, 2020
4 years ago
-
Location Date
November 28, 2022
2 years ago
-
Status Code
700
-
Current Location
TMEG LAW OFFICE 108
Employee Name
POLZER, NATALIE M
-
Attorney Docket Number
0020368
Attorney Name
M. Scott Alprin
Law Office Assigned Location Code
L80
-
Owners
Mark Drawing Code
4000
Mark Identification
VITRIGEL
Case File Statements
- GS0091: Laboratory apparatus and instruments for the exchange of substances and heat; measuring or testing machines and instruments, namely, scales, measuring glassware, test tubes; incubators for laboratory use; cell culture apparatus for laboratory use, namely, cell culture dishes; cell culture apparatus for laboratory use, namely, tissue culture flasks; cell culture apparatus for research use, namely, bioreactor for cell culturing; furniture especially made and used for laboratories, namely, chambers for cell culture for laboratory use; bioreactors for cell culture; cell chips being bio-chips for research or scientific purposes
- B00001: In the Statement, class 10 has been corrected per Restriction dated August 4, 2022, and per fnoted November 28, 2022.
- GS0051: Pharmaceutical preparations for the treatment of wounds and cancer; chemical preparations for medical purposes, namely, treating ophthalmological, otolaryngological and dermatological diseases or disorders; chemical preparations for pharmaceutical purposes for eye irritation tests, corneal permeability tests, skin sensitization tests, vascular permeability tests, liver metabolism and excretion tests and intestine absorption tests; collagen for medical purposes; surgical mesh comprised primarily of living tissue; patches comprising living tissues for skin regeneration; patches comprising living tissues for reinforcement of living tissues; biological tissue cultures for medical purposes; biological tissue cultures for veterinary purposes; transplants, namely, implants comprising living tissues; human allograft tissue; drug delivery agents in the form of sheets, threads and hollow fibers that facilitate the delivery of pharmaceutical preparations; drug delivery agents consisting of compounds that facilitate delivery of a wide range of pharmaceuticals; microcapsules for delivering drugs
- GS0011: Industrial chemicals; chemicals, namely, chemical preparations for scientific laboratory use other than for medical or veterinary purposes; cell culture media for scientific, laboratory and research use, other than for medical or veterinary purposes; microorganism cell culture media for laboratory, scientific and research use, other than for medical or veterinary purposes; cell culture media for scientific or research purposes; cell culture media for laboratory use
- GS0101: Medical apparatus and instruments for use in treating ophthalmological, otolaryngological and dermatological diseases and conditions; artificial skin for medical purposes; artificial skin for surgical purposes; prosthetics or fillings materials, namely, artificial materials for use in the replacement of bones, not for dental use; surgical mesh comprised primarily of artificial materials; suture materials; thread for medical purposes; sutures; hollow [ fibers ] * fiber membranes * for medical use; apparatus for drug delivery system, namely, syringes sold filled with living cells; implantable subcutaneous drug delivery devices sold filled with pharmaceutical preparations for treatment of dermatological disease; prosthetic tissues for parietal use; prosthetic tissues for visceral use; prosthetic tissues for vascular use
Case File Event Statements
-
10/7/2023 - a year ago
28 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
2/5/2023 - 2 years ago
27 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
1/3/2023 - 2 years ago
26 - NOTICE OF UPDATED REGISTRATION CONFIRMATION EMAILED
Type: NURC
-
11/29/2022 - 2 years ago
25 - LIMITATION FROM THE IB EXAMINED AND ENTERED
Type: LIME
-
11/28/2022 - 2 years ago
24 - CORRECTION UNDER SECTION 7 ¿ PROCESSED
Type: COC.
-
8/7/2022 - 2 years ago
23 - LIMITATION OF GOODS RECEIVED FROM IB
Type: LIMG
-
1/30/2021 - 4 years ago
22 - FINAL DECISION TRANSACTION PROCESSED BY IB
Type: FINO
-
12/18/2020 - 4 years ago
21 - GENERIC MADRID TRANSACTION SENT TO IB
Type: XXSS
-
12/18/2020 - 4 years ago
20 - GENERIC MADRID TRANSACTION CREATED
Type: XXCR
-
12/1/2020 - 4 years ago
19 - FINAL DISPOSITION NOTICE CREATED, TO BE SENT TO IB
Type: FICR
-
9/1/2020 - 4 years ago
18 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
6/16/2020 - 4 years ago
17 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
6/16/2020 - 4 years ago
16 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
5/27/2020 - 4 years ago
15 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
5/14/2020 - 5 years ago
14 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
5/13/2020 - 5 years ago
13 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
5/13/2020 - 5 years ago
12 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
5/13/2020 - 5 years ago
11 - ASSIGNED TO LIE
Type: ALIE
-
5/8/2020 - 5 years ago
10 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
2/22/2020 - 5 years ago
9 - REFUSAL PROCESSED BY IB
Type: RFNT
-
2/6/2020 - 5 years ago
8 - NON-FINAL ACTION MAILED - REFUSAL SENT TO IB
Type: RFCS
-
2/5/2020 - 5 years ago
7 - REFUSAL PROCESSED BY MPU
Type: RFRR
-
1/10/2020 - 5 years ago
6 - NON-FINAL ACTION (IB REFUSAL) PREPARED FOR REVIEW
Type: RFCR
-
1/9/2020 - 5 years ago
5 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
1/7/2020 - 5 years ago
4 - APPLICATION FILING RECEIPT MAILED
Type: MAFR
-
1/3/2020 - 5 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
1/3/2020 - 5 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
12/19/2019 - 5 years ago
1 - SN ASSIGNED FOR SECT 66A APPL FROM IB
Type: REPR