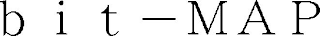Information
-
 Trademark
Trademark
-
 79290762
79290762
-
Serial Number
79290762
-
Registration Number
6337404
-
International Classifications
-
Filing Date
February 06, 2020
6 years ago
-
Registration Date
May 04, 2021
4 years ago
-
Transaction Date
January 19, 2024
2 years ago
-
Status Date
May 04, 2021
4 years ago
-
Published for Opposition Date
February 16, 2021
5 years ago
-
Location Date
May 04, 2021
4 years ago
-
Status Code
700
-
Current Location
PUBLICATION AND ISSUE SECTION
Employee Name
FOSDICK, GEOFFREY A
-
Attorney Docket Number
US-SHUS-027A
Attorney Name
Stacey J. Watson, Esq.
Law Office Assigned Location Code
M20
-
Owners
Mark Drawing Code
5000
Mark Identification
BIT-MAP
Case File Statements
- GS0051: Pharmaceutical preparations for the treatment of allergies, alzheimer disease, adrenocortical insufficiency, allotype pneumonia, alveobronchiolitis, angiocholitis, arthritis, asthmatoid bronchitis, asthma, attention deficit hyperactivity disorder, autism, auto-immune diseases and disorders, autoinflammatory disease, bacteremia, bacterial infection, bacterial pneumonia, bacterial septicemia, bacteroides fragilis, bacteroides thetaiotaomicron, bacteroides uniformis, bacteroides vulgatus, beta thalassemia and myelodysplastic syndromes, and biliary tract cancer, pneumonia, blood disease, blood disorders, bone growth disorders, bone and joint infections, breast cancer, bronchitis, bronchiectasis, cancer, cardiovascular diseases, central nervous system diseases and disorders, cervical cancer, cholecystitis, chronic congestive heart failure, clostridium perfringens, collagen diseases, colorectal cancer, community-acquired pneumonia, congestive heart failure, constipation, crohn's disease, cystitis, deep suppurative disease, dementia, depression, diabetes, dermatologic diseases, duodenal ulcer, dysentery, edematous states, endocarditis, endocrine diseases and disorders, endometrial cancer, endometriosis, endonephritis, enteritis, enterococcus faecalis, epididymitis, epilepsy, esophageal cancer, dysentery, febrile neutropenic infections, gastrointestinal diseases and disorders, gastric cancer, gastric ulcer, genetic diseases and disorders, gonorrhea, graft-versus-host disease, granulocytopenia, gynecological infections, head and neck cancer, heart disease, hematologic disorders, hepatic cirrhosis, hypertension, hyperlipidemi, hypercholesterolemia, hypognathadenitis, incontinence, inflammation and inflammatory diseases, inflammatory bowel disease, intra-abdominal infections, intrauterine infection, irritable bowel syndrome, kidney diseases and disorders, leukemia, liver disorders and diseases, lower respiratory tract infections, lung cancer, lymphoma, melanoma, meningitis, menopausal, metabolic diseases and disorders, multiple myeloma, multiple sclerosis, muscular dystrophy, musculoskeletal diseases and disorders, myeloproliferative diseases, myelofibrosis, nasosinusitis, neoplastic diseases, neurodegenerative diseases and disorders, neurological diseases and disorders, neuromuscular and non-gonococcal urethritis, nosocomial pneumonia, obesity, obsessive-compulsive disorder, oncological diseases and disorders, ophthalmic diseases, osteoarthritis, otitis media, ovarian cancer, pain, pancreatic cancer, parkinson's disease, peptostreptococcus micros, peritonitis and septicemia, pneumonia, polymicrobic infections, prevention of chest pain, prostate cancer, prostatitis, pseudomonas aeruginosa, pyelocystiti, pyelonephritis, reflux sophagitis, renal dysfunction, rheumatic disorders, respiratory diseases, respiratory tract infections, schizophrenia, scleroderma, seizures, septicemia, sexual disfunction, sickle cell disease, skin disorders and diseases, skin and skin structure infections, solid organ transplant rejection, spasms, staphylococcus aureus, streptococcus anginosus, streptococcus intermadius, streptococcus constellatus, stroke, superficial pyogenic infection, suppurative lung disease, syphilis, transmissible alimentary toxicosis, the prevention of organ rejection by renal transplant patients, tumors, ulcerative colitis, urethritis, urinary tract infections, urogenital tract infection, urothelial cancer, vaso-occulisive crisis, viral diseases and disorders, and zollinger-ellison syndrome; pharmaceutical biological preparations for medical purposes for the treatment of allergies, alzheimer disease, adrenocortical insufficiency, allotype pneumonia, alveobronchiolitis, angiocholitis, arthritis, asthmatoid bronchitis, asthma, attention deficit hyperactivity disorder, autism, auto-immune diseases and disorders, autoinflammatory disease, bacteremia, bacterial infection, bacterial pneumonia, bacterial septicemia, bacteroides fragilis, bacteroides thetaiotaomicron, bacteroides uniformis, bacteroides vulgatus, beta thalassemia and myelodysplastic syndromes, biliary tract cancer, pneumonia, blood disease, blood disorders, bone growth disorders, bone and joint infections, breast cancer, bronchitis, bronchiectasis, cancer, cardiovascular diseases, central nervous system diseases and disorders, cervical cancer, cholecystitis, chronic congestive heart failure, clostridium perfringens, collagen diseases, colorectal cancer, community-acquired pneumonia, congestive heart failure, constipation, crohn's disease, cystitis, deep suppurative disease, dementia, depression, diabetes, dermatologic diseases, duodenal ulcer, dysentery, edematous states, endocarditis, endocrine diseases and disorders, endometrial cancer, endometriosis, endonephritis, enteritis, enterococcus faecalis, epididymitis, epilepsy, esophageal cancer, dysentery, febrile neutropenic infections, gastrointestinal diseases and disorders, gastric cancer, gastric ulcer, genetic diseases and disorders, gonorrhea, graft-versus-host disease, granulocytopenia, gynecological infections, head and neck cancer, heart disease, hematologic disorders, hepatic cirrhosis, hypertension, hyperlipidemi, hypercholesterolemia, hypognathadenitis, incontinence, inflammation and inflammatory diseases, inflammatory bowel disease, intra-abdominal infections, intrauterine infection, irritable bowel syndrome, kidney diseases and disorders, leukemia, liver disorders and diseases, lower respiratory tract infections, lung cancer, lymphoma, melanoma, meningitis, menopausal, metabolic diseases and disorders, multiple myeloma, multiple sclerosis, muscular dystrophy, musculoskeletal diseases and disorders, myeloproliferative diseases, myelofibrosis, nasosinusitis, neoplastic diseases, neurodegenerative diseases and disorders, neurological diseases and disorders, neuromuscular and non-gonococcal urethritis, nosocomial pneumonia, obesity, obsessive-compulsive disorder, oncological diseases and disorders, ophthalmic diseases, osteoarthritis, otitis media, ovarian cancer, pain, pancreatic cancer, parkinson's disease, peptostreptococcus micros, peritonitis and septicemia, pneumonia, polymicrobic infections, prevention of chest pain, prostate cancer, prostatitis, pseudomonas aeruginosa, pyelocystiti, pyelonephritis, reflux sophagitis, renal dysfunction, rheumatic disorders, respiratory diseases, respiratory tract infections, schizophrenia, scleroderma, seizures, septicemia, sexual disfunction, sickle cell disease, skin disorders and diseases, skin and skin structure infections, solid organ transplant rejection, spasms, staphylococcus aureus, streptococcus anginosus, streptococcus intermadius, streptococcus constellatus, stroke, superficial pyogenic infection, suppurative lung disease, syphilis, transmissible alimentary toxicosis, the prevention of organ rejection by renal transplant patients, tumors, ulcerative colitis, urethritis, urinary tract infections, urogenital tract infection, urothelial cancer, vaso-occulisive crisis, viral diseases and disorders, and zollinger-ellison syndrome; pharmaceutical preparations for human use, namely, analgesics, antibiotics, anti-fungals, anti-infective preparations, anti-virals, anti-parasitics, and cytokine inhibitory drugs; human growth hormones, immunosuppressants, immunotherapies for the treatment of cancer, medicated dermatological preparations, pharmaceutical antibodies for disease testing, diagnosis, and treatment purposes, pharmaceutical preparations that modulate the immune system, pharmaceutical and biological preparations for immunotherapy, including T Cell therapy, sedatives, smoking cessation preparations, tranquillizers, vaccines for human use, and vitamins; preparations for destroying vermin, fungicides, herbicides
- CC0000: Color is not claimed as a feature of the mark.
- GS0441: Medical screening; providing information relating to medical services; medical testing and physical examination for diagnostic or treatment purposes
- GS0421: Structural and functional analysis of genomes; research and development in the field of microorganisms and cells for the healthcare, scientific, biochemical, pharmaceutical, medical, agricultural, food, cosmetic, animal health, diagnostic, chemical, bioremediation, sanitation, oral care, and oil industries; pharmaceutical research and development; pharmaceutical product evaluation services; medical research; medical laboratory research in the field of genetics; scientific laboratory services in the field of gene expression; scientific research relating to genetics
Case File Event Statements
-
1/19/2024 - 2 years ago
27 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
9/5/2021 - 4 years ago
26 - FINAL DECISION TRANSACTION PROCESSED BY IB
Type: FINO
-
8/12/2021 - 4 years ago
25 - FINAL DISPOSITION NOTICE SENT TO IB
Type: FICS
-
8/12/2021 - 4 years ago
24 - FINAL DISPOSITION PROCESSED
Type: FIMP
-
8/4/2021 - 4 years ago
23 - FINAL DISPOSITION NOTICE CREATED, TO BE SENT TO IB
Type: FICR
-
5/4/2021 - 4 years ago
22 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
2/16/2021 - 5 years ago
21 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
2/16/2021 - 5 years ago
20 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
1/27/2021 - 5 years ago
19 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
1/8/2021 - 5 years ago
18 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
1/8/2021 - 5 years ago
17 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
1/8/2021 - 5 years ago
16 - NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED
Type: GNEN
-
1/8/2021 - 5 years ago
15 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
1/8/2021 - 5 years ago
14 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
1/7/2021 - 5 years ago
13 - APPLICANT/CORRESPONDENCE CHANGES (NON-RESPONSIVE) ENTERED
Type: CHAN
-
1/7/2021 - 5 years ago
12 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
1/7/2021 - 5 years ago
11 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
1/7/2021 - 5 years ago
10 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
1/7/2021 - 5 years ago
9 - TEAS CHANGE OF OWNER ADDRESS RECEIVED
Type: COAR
-
9/7/2020 - 5 years ago
8 - NON-FINAL ACTION MAILED - REFUSAL SENT TO IB
Type: RFCS
-
9/3/2020 - 5 years ago
7 - REFUSAL PROCESSED BY MPU
Type: RFRR
-
8/7/2020 - 5 years ago
6 - APPLICATION FILING RECEIPT MAILED
Type: MAFR
-
8/4/2020 - 5 years ago
5 - NON-FINAL ACTION (IB REFUSAL) PREPARED FOR REVIEW
Type: RFCR
-
8/3/2020 - 5 years ago
4 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
8/3/2020 - 5 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
8/3/2020 - 5 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
7/30/2020 - 5 years ago
1 - SN ASSIGNED FOR SECT 66A APPL FROM IB
Type: REPR