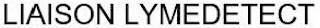Information
-
 Trademark
Trademark
-
 79300833
79300833
-
Serial Number
79300833
-
Registration Number
6532877
-
International Classifications
- 1 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry
- 5 - Pharmaceutical and veterinary preparations
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
- 10 - Surgical, medical, dental and veterinary apparatus and instruments, artificial limbs, eyes and teeth
-
Filing Date
August 26, 2020
4 years ago
-
Registration Date
October 26, 2021
3 years ago
-
Transaction Date
May 09, 2022
2 years ago
-
Status Date
October 26, 2021
3 years ago
-
Published for Opposition Date
August 10, 2021
3 years ago
-
Location Date
October 26, 2021
3 years ago
-
Status Code
700
-
Current Location
PUBLICATION AND ISSUE SECTION
Employee Name
MASON, JARED MICHAEL
-
Attorney Name
Jeffrey B. Sladkus, Esq.
Law Office Assigned Location Code
N10
-
Owners
Mark Drawing Code
4000
Mark Identification
LIAISON LYMEDETECT
Case File Statements
- GS0091: Scientific apparatus and instruments for measuring relative DNA, RNA and protein and parts and fittings therefor; laboratory apparatus and instruments for conducting and evaluating diagnostic examinations for medical purposes, and parts and fittings therefor, namely, microscopes, microscope slides; diagnostic instruments for scientific purposes in the nature of diagnostic apparatus for testing food; diagnostic testing apparatus for scientific and research testing being diagnostic apparatus for testing food; computer systems comprising of computer hardware and downloadable computer software for collecting, storing, analysing and reporting biological information, and for sample tracking and managing projects, laboratory workflows and data, for use in the fields of scientific, diagnostic and clinical research and for clinical diagnostic purposes; downloadable software for use with electronic medical and diagnostic apparatus; downloadable software and downloadable software containing algorithms, in particular downloadable computer databases, for helping to make medical decisions or carry out research, for diagnostic and therapeutic applications; computer hardware for medical and diagnostic tasks; devices in the nature of computer hardware for generating images for detecting, quantifying and linking biological or chemical molecules; liquid analyzers; laboratory instruments, namely, genome sequencers being laboratory devices for detecting genetic sequences; specimen containers, namely, petri dishes
- GS0051: Diagnostic preparations for medical purposes; diagnostic substances for medical purposes; diagnostic reagents for use in diagnostic tests for medical purposes; chemical preparations for testing blood for medical purposes; chemical preparations for testing blood for veterinary purposes; in vitro diagnostic preparations for medical purposes; in vitro diagnostic preparations for veterinary purposes; chemicals reagents for testing DNA for medical purposes; nucleic acid sequences for medical and veterinary purposes; diagnostic reagents for use in diagnostic tests for veterinary purposes; reactants for medical diagnosis being diagnostic preparations for medical purposes; clinical medical diagnostic reagents; diagnostic biomarker reagents for medical purposes; diagnostic biomarker reagents for veterinary purposes; indicators for medical diagnosis being diagnostic preparations for medical purposes; diagnostic substances for medical use; diagnostic substances for veterinary use; medicated antiserums for diagnostic purposes for diagnosing skin conditions; chemical test reagents for medical purposes; chemical test reagents for veterinary purposes; reagents for microbiological analysis for medical purposes; reagents for microbiological analysis for veterinary purposes; reagents for use with analyzers for medical purposes; reagents for use with analyzers for veterinary purposes; diagnostic chemical reagents for medical use; diagnostic chemical reagents for veterinary use; medical diagnostic reagents and assays for testing of body fluids; diagnostic agents for pharmaceutical use; test strips for measuring blood glucose levels; medical diagnostic test strips for monitoring blood sugar levels; medical diagnostic kits comprising diagnostic reagents for extracting, testing and analyzing body fluids, nucleic acids, DNA, RNA; medical diagnostic kits comprising diagnostic reagents for extracting, isolating, testing and analyzing blood cells; test strips containing medical diagnostic reagents for measuring blood glucose levels
- GS0011: Diagnostic preparations for scientific or research use; diagnostic reagents and chemical reagents, for laboratory or research use, not for medicinal purposes; blood glucose control solutions used in diagnostic test kits, for laboratory or research use; diagnostic test kits consisting primarily of monoclonal antibodies, buffers, and reagents to monitor toxicity of drugs for laboratory use; scientific chemical products for diagnostic use in the nature of chemical test paper; chemical and reagent solutions being chemical reagents used in diagnostic test kits, not for medical purposes; diagnostic biomarker reagents for analytical, research and scientific use; assays and reagents for use in genetic research; chemically treated test strips, other than for medical purposes for testing the pH of liquids; chromatography chemicals for laboratory use; reagents for use in scientific apparatus for chemical or biological analysis for testing the presence of antigens in blood, human serum, plasma, urine, other biological fluids, and tissue; chemically treated non-medical test strips for detecting alcohol and drugs
- GS0101: Kits for medical diagnostic applications in the nature of medical test kits for diabetes monitoring for home use; apparatus for carrying out medical diagnoses, in particular fluorescence diagnostic apparatus and instruments used for the purpose of identifying infectious diseases and the severity thereof being medical diagnostic apparatus for detecting cancer; electromagnetic medical diagnostic imaging apparatus for medical use; electromagnetic medical diagnostic imaging apparatus for medical purposes containing fluorimeters used to register fluorescence signals and produce data; diagnostic lasers for medical purposes; medical and diagnostic devices, apparatus and/or equipment for molecular analysis being medical diagnostic apparatus for testing DNA and RNA samples; scanners for medical diagnosis, namely, x-ray CT scanners; blood spectrum analyzers for use in medical diagnosis; ultrasonic medical diagnostic apparatus; magnetic field generators for medical purposes, namely, magnetic resonance imaging apparatus for medical purposes; electromagnetic medical diagnostic imaging apparatus; optical instruments for medical diagnostics in the nature of optometric instruments for locating the optical center of ophthalmic lenses; containers for sample analysis, syringes and plungers being containers for medical waste; sampling vials of plastic or other materials being blood sampling tubes; preparation vials of plastic or other materials being blood sampling tubes; devices containing reagents for medical diagnostic test use, namely, capillary reagent tubes; test devices containing chemical reagents for in-vitro diagnostic medical testing being sample preparation device for medical diagnostic uses
- D10000: "LYME DETECT"
Case File Event Statements
-
5/9/2022 - 2 years ago
30 - FINAL DECISION TRANSACTION PROCESSED BY IB
Type: FINO
-
4/19/2022 - 2 years ago
29 - FINAL DISPOSITION NOTICE SENT TO IB
Type: FICS
-
4/19/2022 - 2 years ago
28 - FINAL DISPOSITION PROCESSED
Type: FIMP
-
1/26/2022 - 3 years ago
27 - FINAL DISPOSITION NOTICE CREATED, TO BE SENT TO IB
Type: FICR
-
10/26/2021 - 3 years ago
26 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
8/15/2021 - 3 years ago
25 - NOTIFICATION PROCESSED BY IB
Type: GPNX
-
8/10/2021 - 3 years ago
24 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
8/10/2021 - 3 years ago
23 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
7/28/2021 - 3 years ago
22 - NOTIFICATION OF POSSIBLE OPPOSITION SENT TO IB
Type: OPNS
-
7/28/2021 - 3 years ago
21 - NOTICE OF START OF OPPOSITION PERIOD CREATED, TO BE SENT TO IB
Type: OP2R
-
7/21/2021 - 3 years ago
20 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
7/1/2021 - 3 years ago
19 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
6/22/2021 - 3 years ago
18 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
6/21/2021 - 3 years ago
17 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
6/21/2021 - 3 years ago
16 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
6/15/2021 - 3 years ago
15 - APPLICANT/CORRESPONDENCE CHANGES (NON-RESPONSIVE) ENTERED
Type: CHAN
-
6/15/2021 - 3 years ago
14 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
6/15/2021 - 3 years ago
13 - TEAS CHANGE OF DOMESTIC REPRESENTATIVES ADDRESS
Type: ECDR
-
6/15/2021 - 3 years ago
12 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
6/15/2021 - 3 years ago
11 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
6/15/2021 - 3 years ago
10 - TEAS CHANGE OF OWNER ADDRESS RECEIVED
Type: COAR
-
2/14/2021 - 4 years ago
9 - REFUSAL PROCESSED BY IB
Type: RFNT
-
1/25/2021 - 4 years ago
8 - NON-FINAL ACTION MAILED - REFUSAL SENT TO IB
Type: RFCS
-
1/25/2021 - 4 years ago
7 - REFUSAL PROCESSED BY MPU
Type: RFRR
-
1/5/2021 - 4 years ago
6 - NON-FINAL ACTION (IB REFUSAL) PREPARED FOR REVIEW
Type: RFCR
-
1/4/2021 - 4 years ago
5 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
1/1/2021 - 4 years ago
4 - APPLICATION FILING RECEIPT MAILED
Type: MAFR
-
12/28/2020 - 4 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
12/28/2020 - 4 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM
Type: NWOS
-
12/24/2020 - 4 years ago
1 - SN ASSIGNED FOR SECT 66A APPL FROM IB
Type: REPR