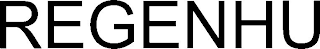Information
-
 Trademark
Trademark
-
 79322244
79322244
-
Serial Number
79322244
-
Registration Number
7134865
-
International Classifications
- 1 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry
- 5 - Pharmaceutical and veterinary preparations
- 7 - Machines and machine tools
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
- 10 - Surgical, medical, dental and veterinary apparatus and instruments, artificial limbs, eyes and teeth
- 41 - Education
- 42 - Scientific and technological services and research and design relating thereto
-
Filing Date
April 30, 2021
4 years ago
-
Registration Date
August 15, 2023
2 years ago
-
Transaction Date
November 15, 2023
2 years ago
-
Status Date
August 15, 2023
2 years ago
-
Published for Opposition Date
May 30, 2023
2 years ago
-
Location Date
August 15, 2023
2 years ago
-
Status Code
700
-
Current Location
PUBLICATION AND ISSUE SECTION
Employee Name
BETTS, MARCYA N
-
Attorney Docket Number
RGU2201USTM1
Attorney Name
Brian P. Sullivan
Law Office Assigned Location Code
L60
-
Owners
Mark Drawing Code
4000
Mark Identification
REGENHU
Case File Statements
- GS0411: Training and education services in the nature of workshops, seminars, non-downloadable webinars, and conferences in the field of bioprinting and biomanufacturing
- GS0091: laboratory apparatus and instruments, including accessories, namely, bioreactors, for cell culturing; laboratory apparatus and instruments for engineering biological tissues, tissue compositions, cell aggregates and cells, namely, laboratory apparatus and instruments for the design, engineering and maintenance of biological tissues, tissue compositions, cell aggregates and cells; laboratory robots that can be used in biotechnology and in the field of pharmaceutical and cosmetic products; 2D and 3D scanners in the field of additive manufacturing processes and applications; optical apparatus and instruments, namely, cameras, microscopes, laser measuring systems, calibration apparatus for calibrating electric microvalves, and measurement apparatus being temperature indicators, flowmeters, distance measuring apparatus, calipers, all in the field of additive manufacturing processes and applications; optical readers and optical inspection apparatus in the field of additive manufacturing processes and applications; laboratory tools and accessories for the manufacture and production of biological tissues, especially for observation, measurement, handling, stimulation or modification of biological tissues, tissue compositions, cell aggregates and cells, namely, cameras, electric sensors, optical profilers, electrical transducers, thermal control being thermal printers, downloadable software and pre-installed software for 2D and 3D design for the definition and management of manufacturing and production processes for biological tissues or organs, medical implants and pharmaceuticals; downloadable software and pre-installed software for the operation and control of machines, instruments and apparatus intended for engineering, manufacturing and producing biological tissues, tissue compositions, cell aggregates, cells, organs, medical implants or pharmaceuticals; downloadable software and pre-installed software for the operation and control of devices being used in biotechnology and in the pharmaceutical and cosmetic industries for computer-aided three-dimensional dosing of biomaterials for biofunctional shaping of cell carrier structures, tissues, blood vessels, implants, prostheses and drug delivery systems; downloadable software and pre-installed software used for 3D design, 3D printing and 3D manufacturing; downloadable software and pre-installed software for image processing and medical image processing
- GS0071: Machines and their parts and equipment, namely, 3D printers, 3D bioprinters, handling apparatus for loading and unloading in the nature of loading and unloading machines, and automatic handling machine, all for manufacturing and producing biological tissues and organs, medical implants, pharmaceuticals; machines and instruments, namely, 3D printers, 3D bioprinters, handling apparatus for loading and unloading in the nature of loading and unloading machines, and automatic handling machine, all for manufacturing and assembling biological tissues, tissue compositions, cell aggregates and cells; additive manufacturing machines for manufacturing and producing 3D objects; extruders, namely, extruding machines for microextrusion, multi-axial extrusion, droplet extrusion, micro-fiber extrusion, patterning, mixing, combination, and 2D and 3D arrangement of liquids, biological compounds, cell solutions, hydrogels, hydrogels loaded with cells, bioceramics, bioplastics, base materials for micro and nano particles; cartridges for containing printing material for use with 3D printers, sold empty; cartridges for containing bioprinting materials for use with 3D bioprinters, sold empty; printing cartridges for containing biological materials for use with 3D printers and bioprinters, sold empty; printing cartridges for containing ink for 3D printing of biological tissues and organs; tools and accessories for manufacturing and producing biological tissues, especially for observation, measuring, handling, stimulation or modification of biological tissues, tissue compositions, cell aggregates and cells, namely, 3D printers, and thermal control being block type thermal power stations for power generation
- GS0051: ATMPs (Advanced Therapy Medicinal Products), namely, medicines for human use based on genes, tissues or cells for the treatment of cancer, degenerative diseases, bacterial infections, viral infections, autoimmune diseases, and traumatic injuries; biological tissues, namely, bone, cartilage, skin, blood vessel, neural, muscular, heart, adipose, ocular, liver, pancreatic, and gut tissues and biological organ tissue manufactured for medical and transplant purposes; biological tissue samples being biological tissue cultures, biological tissue-engineered tissues being biological tissue cultures, tissue compositions being biological tissue grafts, cell aggregates and cells intended for medical use for medical applications; medical implants comprising living bioactives, biological cells or tissues; biological preparations for the treatment of cancer, degenerative diseases, bacterial infections, viral infections, autoimmune diseases, and traumatic injuries and biological tissue cultures for medical purposes; biological preparations for the treatment of cancer, degenerative diseases, bacterial infections, viral infections, autoimmune diseases, and traumatic injuries and biological tissue cultures for veterinary purposes; pharmaceutical products for the treatment of cancer, degenerative diseases, bacterial infections, viral infections, autoimmune diseases, and traumatic injuries; tissue-regenerative pharmaceutical preparations for prevention and treatment of cancer, degenerative diseases, bacterial infections, viral infections, autoimmune diseases, and traumatic injuries; medicines and gel capsules for medical use for the treatment of cancer, degenerative diseases, bacterial infections, viral infections, autoimmune diseases, and traumatic injuries; biological implants for surgical and orthopedic purposes, namely, bone, cartilage, skin, blood vessel, neural, muscular, heart, adipose, ocular, liver, pancreatic, gut, and biological organ tissue intended for subsequent implantation
- GS0011: Chemical, biochemical and biological products, namely, unprocessed natural and synthetic polymers, hydrogels being cellulose for industrial purposes and collagen for industrial purposes, weak and strong gels being Polyethylene glycol, synthetic unprocessed resins, pastes being silicone fluids, degreasing solvents, liquid carriers being cell culture media, chemical additives improving ink printability, plasticizers, biochemicals, unprocessed growth factors, thermoplastic polymers, chemical composites, metallic and ceramic micro and nanoparticles being calcium phosphates, graphene, iron oxide, and silica particles, biological molecules being enzymes and peptides, active pharmaceutical ingredients and cell culture media for laboratory use, all for use in the manufacture and production of biological tissues; ink for 3D printing of biological tissues and organs; biologically compatible materials, namely, hydrogels used to create structures and scaffolds used in the manufacture and production of biological tissues; biologically compatible materials, namely, hydrogels used to create polymer arrays for scientific and medical research used in the manufacture and production of biological tissues; cell culture media, reagents for scientific or medical research use for use with biological tissues, tissue arrays, cell aggregates and cells; biological tissue samples being biological tissue cultures, biological tissue-engineered tissues being biological tissue cultures, tissue arrays, cell aggregates and cells for scientific research purpose; liquid photosensitive chemicals and resin products, namely, photoinitiators for use in the manufacture of 3D objects; solid imaging materials used to create 3D objects, namely, photocurable unprocessed polymers, unprocessed powder polymers and plastic molding compounds used in the manufacture of 3D objects; unprocessed polymers and unprocessed artificial resins for additive manufacturing of crowns, being dental inlays and onlays, and dental implants
- GS0101: Non-biological implants being surgical implant comprised of artificial materials produced by additive manufacturing intended for surgical and orthopedic purposes; dental crowns, incrustations being dental inlays and onlays and dental implants made by additive manufacturing; artificial implants and transplants being implants comprising natural, non-living material intended for replacing and/or supporting the function of missing or damaged body parts; surgical apparatus and instruments involving the extrusion, dosing or manufacture of biological tissues, tissue arrays, cell aggregates and cells, namely, surgical robots and surgical instruments; surgical and medical devices for use in situ bioprinting, namely, surgical robots and surgical instruments
- GS0421: Scientific services, namely, conducting technical project studies in the nature of conducting scientific feasibility studies in the field of additive manufacturing, technological research in the field of additive manufacturing, research and development of new products for others in the field of additive manufacturing; research and development in the field of additive manufacturing; scientific services in connection with the design, development and support of software, namely, conducting technical project studies in the nature of conducting scientific feasibility studies, computer software consultancy, computer software design, research and development of new products for others; advice and information concerning additive manufacturing research and development and software design and development
Case File Event Statements
-
11/15/2023 - 2 years ago
39 - FINAL DISPOSITION NOTICE SENT TO IB
Type: FICS
-
11/15/2023 - 2 years ago
38 - FINAL DISPOSITION PROCESSED
Type: FIMP
-
11/15/2023 - 2 years ago
37 - FINAL DISPOSITION NOTICE CREATED, TO BE SENT TO IB
Type: FICR
-
8/15/2023 - 2 years ago
36 - NOTICE OF REGISTRATION CONFIRMATION EMAILED
Type: NRCC
-
8/15/2023 - 2 years ago
35 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
5/30/2023 - 2 years ago
34 - NOTIFICATION PROCESSED BY IB
Type: GPNX
-
5/30/2023 - 2 years ago
33 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
5/30/2023 - 2 years ago
32 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
5/10/2023 - 2 years ago
31 - NOTIFICATION OF POSSIBLE OPPOSITION SENT TO IB
Type: OPNS
-
5/10/2023 - 2 years ago
30 - NOTICE OF START OF OPPOSITION PERIOD CREATED, TO BE SENT TO IB
Type: OP2R
-
5/10/2023 - 2 years ago
29 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
4/26/2023 - 2 years ago
28 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
4/25/2023 - 2 years ago
27 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
4/25/2023 - 2 years ago
26 - NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED
Type: GNEN
-
4/25/2023 - 2 years ago
25 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
4/25/2023 - 2 years ago
24 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
3/29/2023 - 2 years ago
23 - NOTIFICATION OF POSSIBLE OPPOSITION - PROCESSED BY IB
Type: OPNX
-
3/8/2023 - 2 years ago
22 - NOTIFICATION OF POSSIBLE OPPOSITION SENT TO IB
Type: OPNS
-
3/8/2023 - 2 years ago
21 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
3/8/2023 - 2 years ago
20 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
3/8/2023 - 2 years ago
19 - TEAS REQUEST FOR RECONSIDERATION RECEIVED
Type: ERFR
-
3/8/2023 - 2 years ago
18 - NOTIFICATION OF POSSIBLE OPPOSITION CREATED, TO BE SENT TO IB
Type: OPNR
-
12/18/2022 - 2 years ago
17 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
9/8/2022 - 3 years ago
16 - NOTIFICATION OF FINAL REFUSAL EMAILED
Type: GNFN
-
9/8/2022 - 3 years ago
15 - FINAL REFUSAL E-MAILED
Type: GNFR
-
9/8/2022 - 3 years ago
14 - FINAL REFUSAL WRITTEN
Type: CNFR
-
8/3/2022 - 3 years ago
13 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
8/2/2022 - 3 years ago
12 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
8/2/2022 - 3 years ago
11 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
6/10/2022 - 3 years ago
10 - NEW REPRESENTATIVE AT IB RECEIVED
Type: NREP
-
2/20/2022 - 3 years ago
9 - REFUSAL PROCESSED BY IB
Type: RFNT
-
2/2/2022 - 3 years ago
8 - NON-FINAL ACTION MAILED - REFUSAL SENT TO IB
Type: RFCS
-
2/2/2022 - 3 years ago
7 - REFUSAL PROCESSED BY MPU
Type: RFRR
-
1/12/2022 - 3 years ago
6 - NON-FINAL ACTION (IB REFUSAL) PREPARED FOR REVIEW
Type: RFCR
-
1/11/2022 - 3 years ago
5 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
1/3/2022 - 3 years ago
4 - ASSIGNED TO EXAMINER
Type: DOCK
-
10/16/2021 - 4 years ago
3 - APPLICATION FILING RECEIPT MAILED
Type: MAFR
-
10/12/2021 - 4 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
10/8/2021 - 4 years ago
1 - SN ASSIGNED FOR SECT 66A APPL FROM IB
Type: REPR