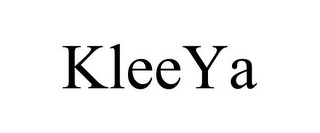Information
-
 Trademark
Trademark
-
 87436630
87436630
-
Serial Number
87436630
-
Registration Number
5548392
-
International Classifications
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
- 10 - Surgical, medical, dental and veterinary apparatus and instruments, artificial limbs, eyes and teeth
- 37 - Building construction
- 42 - Scientific and technological services and research and design relating thereto
-
Filing Date
May 04, 2017
8 years ago
-
Registration Date
August 28, 2018
7 years ago
-
Transaction Date
February 04, 2025
12 months ago
-
Status Date
February 03, 2025
12 months ago
-
Published for Opposition Date
June 12, 2018
7 years ago
-
Location Date
February 03, 2025
12 months ago
-
Status Code
702
-
Current Location
Historical data usage
Employee Name
POLLACK, ALISON
-
Attorney Docket Number
STR003UST
Attorney Name
Edmund J. Ferdinand, III
-
Owners
Mark Drawing Code
4
Mark Identification
KLEEYA
Case File Statements
- GS0091: Diagnostic apparatus, not for medical purposes, namely, for determining and analyzing the composition, compounds and temperature of semi-liquids, gases and liquids and for processing the mixture of semi-liquids, gases and liquids, all for laboratory use; [ Dosimeters; Digital indicators; ] Pressure sensors; Liquid level switches for monitoring and controlling liquids in tanks and vessels; Sensors and detectors for analyzing and determining the composition, compounds and temperature of gases and liquids and for processing the mixture of gases and liquids; [ electronic process monitors for monitoring flowmeters; ] Liquid level monitoring apparatus, namely, liquid level sensors for monitoring liquids in tanks and vessels; [ Monitoring apparatus, automated electric liquid analyzer for monitoring a condition in body fluid samples in an individual for laboratory use; ] Diagnostic detection apparatus, for the detection of pathogens for laboratory or research use or for detecting genetic sequences; Optical sensors; Electronic data loggers and recorders; [ Barometers; Gas, liquid and semi-liquid pressure meters; Pressure measuring apparatus; Pressure controllers for controlling the pressure of liquids, semi-liquid, and gaseous substances in industrial processes; Pressure indicators; Mass flow meters; Flow measuring apparatus, namely, flowmeters; Sound level meters; ] Instruments for measuring levels of fluids, namely, level gauges, level sensors and liquid level sensors, not for medical use; Liquid level meters; Level indicators; Volumetric measuring apparatus, namely, electronic metering systems comprised primarily of flow meters and controlled volume pumps for metering the volume of liquids, semi-liquids and gases in laboratory analyzer systems; [ Pressure regulators for laboratory or research use; ] Flow regulators for laboratory or research use; Electric control apparatus, namely, electronic controllers for automated analyzer systems comprised primarily of automated liquid and gas analyzers for regulating the flow of gases, liquids and semi-liquids; Electronic process controlling apparatus, namely, industrial process control software; Programmable control apparatus, namely, programmable electronic controllers for controlling the pressure of liquids, semi-liquids and gaseous substances in commercial and industrial use; Laboratory chemical reactors; [ Hollow glass containers for laboratory use, being laboratory glassware; Retorts for use in high temperature chemical reactions; Glassware for use in a laboratory; ] Holders specially adapted for laboratory test tubes; Incubators for laboratory use; Laboratory instruments, namely, automated liquid analyzer systems being automated analyzers, controlled volume pumps, electronic pipettes for laboratory use; [ Pipet jars specially adapted for pipettes; ] Pipette tips being laboratory consumables; [ Pipettes; Data storage media, namely, blank digital storage media and blank electronic storage media; Information storage apparatus, namely, blank digital storage media and blank electronic storage media; ] Data processors; Data recorded electronically, namely, electronic data recorders; Computer databases, namely, electronic databases in the field of managing patient medical information recorded on computer media; Recorded computer software for operating automated analyzer systems in laboratory and industrial use; Computer software for use in operating medical decision support systems; Data integrated recorded computer software for the collection, editing, organizing, modifying, book marking, transmission, and storage of data and information related to chemical analysis apparatus for laboratory or industrial use; Lab units for the mixing of liquids, namely, automated analyzers being automated liquid analyzers, namely, flasks and vessels for laboratory use; Lab units for the dilution of liquids, namely, automated analyzer systems being automated liquid analyzers, pipettes and controlled volume pumps; Lab units for the transfer of liquids, namely, laboratory storage tubes, flasks, glassware bottles, pipette tips and controlled volume pumps
- GS0371: Maintenance of medical equipment; Repair or maintenance of medical machines and apparatus; Servicing of medical equipment in the nature of medical imaging apparatus and automated analyzer systems both used in medical laboratory imaging centers
- GS0101: [ Medical apparatus for administering intravenous fluid solutions, namely, fluid warmers; ] Automatic analyzers for medical diagnosis, namely, the pathological condition in body fluid samples; [ Automatic medical apparatus for dosing humans by injection, namely, drug delivery devices and systems by injection; ] Automated medical diagnostic analysers for body fluids for medical purposes; [ Containers for medical use, namely, containers for body fluid samples, namely, capillary tubes and vessels being capillary tubes for blood and samples and capillary reagent tubes; ] Diagnostic, examination, and monitoring equipment, namely, automated analyzer systems for medical use comprised primarily of medical diagnostic instruments for the analysis of body fluids; Diagnostic apparatus for medical purposes for testing the condition in body fluid samples; [ Diagnostic imaging apparatus for medical use, namely, MRI diagnostic apparatus, ultrasonic diagnostic apparatus, X-ray diagnostic apparatus; ] Diagnostic measuring apparatus for medical use, namely, medical diagnostic instruments for measuring the condition in body fluid samples; Fluid collection containers for medical use; [ Breath gas analyzers for medical diagnostic analysis; ] Apparatus for blood analysis; Apparatus for analysing substances, namely, medical diagnostic instruments for analyzing the condition in body fluid samples; [ Medical apparatus and instruments for obtaining body fluid samples; Medical specimen cups; Medical tubing for administering drugs; ] Measuring instruments adapted for medical use, namely, devices for measuring body fluid sample conditions and properties; [ Patient monitoring instruments, namely, patient monitoring sensors and alarms and patient medical monitors for monitoring body fluid sample properties; Physical analyzers for medical use, namely, hematology analyzers for medical diagnostic use; Pipetting instruments for medical use, namely, dropping pipettes for medical purposes; ] Specimen samplers for medical use, namely, sample preparation device for medical diagnostic uses, medical devices for obtaining body fluid samples, blood sampling tubes; [ Infusion pumps for medical use in dispensing measured amounts of pharmaceuticals from containers into the bloodstream over time; ] Temperature measuring instruments for medical use, namely, electronic temperature monitors for medical use [ ; Capillary tubes, namely, capillary tubes for bloods and samples ]
- GS0421: Design of mechanical, electromechanical and optoelectronic apparatus and instruments; Creation of control programs for automated measurement, assembly, adjustment, and related visualisation; Creation, maintenance and updating software; Technical writing, namely, preparation of technical manuals; Services for the provision of technology information in the field of automated analyzer in laboratory and medical use; Professional information technology consultancy in the field of automated analyzer systems for laboratory and medical use; Inspection of medical equipment and automated analyzer systems for quality control purposes; Installation of computer firmware; Calibration of instruments, Calibration services relating to analytical apparatus, Calibration services relating to electronic apparatus, Design of software for digital signal processing
Case File Event Statements
-
5/8/2017 - 8 years ago
1 - NEW APPLICATION ENTERED
Type: NWAP
-
8/3/2017 - 8 years ago
4 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
5/9/2017 - 8 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
8/2/2017 - 8 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
2/3/2018 - 8 years ago
7 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
3/6/2018 - 7 years ago
11 - FINAL REFUSAL WRITTEN
Type: CNFR
-
3/6/2018 - 7 years ago
12 - FINAL REFUSAL E-MAILED
Type: GNFR
-
3/6/2018 - 7 years ago
13 - NOTIFICATION OF FINAL REFUSAL EMAILED
Type: GNFN
-
4/26/2018 - 7 years ago
14 - TEAS REQUEST FOR RECONSIDERATION RECEIVED
Type: ERFR
-
8/3/2017 - 8 years ago
5 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
8/3/2017 - 8 years ago
6 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
2/12/2018 - 7 years ago
8 - ASSIGNED TO LIE
Type: ALIE
-
2/14/2018 - 7 years ago
9 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
2/14/2018 - 7 years ago
10 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
5/4/2018 - 7 years ago
17 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
6/12/2018 - 7 years ago
24 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
4/30/2018 - 7 years ago
15 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
4/30/2018 - 7 years ago
16 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
5/4/2018 - 7 years ago
18 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
5/4/2018 - 7 years ago
19 - NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED
Type: GNEN
-
5/4/2018 - 7 years ago
20 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
5/4/2018 - 7 years ago
21 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
5/23/2018 - 7 years ago
22 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
6/12/2018 - 7 years ago
23 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
8/28/2018 - 7 years ago
25 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
7/15/2019 - 6 years ago
26 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
4/7/2021 - 4 years ago
29 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
4/7/2021 - 4 years ago
30 - TEAS WITHDRAWAL OF ATTORNEY RECEIVED-FIRM RETAINS
Type: EWAF
-
4/7/2021 - 4 years ago
32 - APPLICANT/CORRESPONDENCE CHANGES (NON-RESPONSIVE) ENTERED
Type: CHAN
-
4/7/2021 - 4 years ago
27 - TEAS CHANGE OF OWNER ADDRESS RECEIVED
Type: COAR
-
4/7/2021 - 4 years ago
28 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
4/7/2021 - 4 years ago
31 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
9/21/2022 - 3 years ago
33 - AUTOMATIC UPDATE OF ASSIGNMENT OF OWNERSHIP
Type: ASGN
-
8/28/2023 - 2 years ago
34 - COURTESY REMINDER - SEC. 8 (6-YR) E-MAILED
Type: REM1
-
2/1/2024 - 2 years ago
35 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
2/1/2024 - 2 years ago
36 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
8/12/2024 - a year ago
38 - TEAS SECTION 8 & 15 RECEIVED
Type: E815
-
1/8/2025 - a year ago
39 - CASE ASSIGNED TO POST REGISTRATION PARALEGAL
Type: APRE
-
2/3/2025 - 12 months ago
40 - REGISTERED - SEC. 8 (6-YR) ACCEPTED & SEC. 15 ACK.
Type: C15A
-
2/3/2025 - 12 months ago
41 - NOTICE OF ACCEPTANCE OF SEC. 8 & 15 - E-MAILED
Type: NA85
-
2/1/2024 - 2 years ago
37 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA