Information
-
 Trademark
Trademark
-
 88028445
88028445
-
Serial Number
88028445
-
Registration Number
6008686
-
International Classifications
- 1 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry
- 5 - Pharmaceutical and veterinary preparations
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
- 42 - Scientific and technological services and research and design relating thereto
-
Filing Date
July 06, 2018
7 years ago
-
Registration Date
March 10, 2020
5 years ago
-
Transaction Date
March 10, 2025
7 months ago
-
Status Date
March 10, 2020
5 years ago
-
Published for Opposition Date
June 18, 2019
6 years ago
-
Location Date
February 04, 2020
5 years ago
-
First Use Anywhere Date
November 08, 2019
5 years ago
-
First Use In Commerce Date
November 08, 2019
5 years ago
-
Status Code
700
-
Current Location
PUBLICATION AND ISSUE SECTION
Employee Name
BRADLEY, EVELYN
-
Attorney Docket Number
107541-0038
Attorney Name
Rachelle A. Dubow, Esq.
Law Office Assigned Location Code
L40
-
Owners
Case File Statements
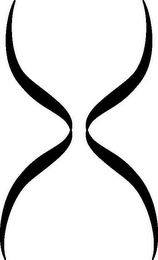
- DM0000: The mark consists of a stylized helix design.
- GS0091: Gene therapy technology in the nature of downloadable software for gene-editing that uses de novo-engineered gene-editing proteins expressed with synthetic mRNA; cell therapy products, namely, cell reprogramming technology in the nature of instruments for scientific laboratory research that use reprogramming factors expressed with synthetic mRNA, namely, cell-culture instruments in the nature of cell incubators, cell transfection instruments, and cell imaging instruments
- GS0051: Pharmaceuticals for treating human and veterinary non-infectious diseases in the nature of inflammatory diseases, infectious diseases, vitamin B12 deficiency, degenerative joint diseases, sensory organ diseases, hereditary diseases, namely, genetic diseases in the nature of chromosomal abnormalities, single gene defects, multifactorial and teratogenic conditions, idiopathic intracranial hypertension, aesthetic skin diseases, traumatic brain injury, psychological diseases and physiological diseases in the nature of heart disease; gene therapy products, namely, genetically engineered cells and tissues for medical purposes, specifically for transplant purposes; chemical reagents for medical and veterinary purposes; synthetic mRNA being nucleic acid sequences and chemical reagents for medical and veterinary purposes for correcting deficient or defective genes; cell therapy products, namely, pharmaceutical preparations for use in cell therapy for treatment of cancer; synthetic mRNA being nucleic acid sequences and related chemical reagents for medical and veterinary purposes for reprogramming cells; synthetic RNA encoding reprogramming factors, namely, pharmaceutical preparations for use in cell therapy for treatment of wounded skin, cancer, chromosomal abnormalities, single gene defects, multifactorial and teratogenic conditions, idiopathic intracranial hypertension, aesthetic skin diseases, traumatic brain injury, and heart disease; products in the nature of pharmaceutical preparations for use in cell therapy for converting non-pluripotent cells into pluripotent stem cells, with or without gene-editing, for treatment of wounded skin, cancer, chromosomal abnormalities, single gene defects, multifactorial and teratogenic conditions, idiopathic intracranial hypertension, aesthetic skin diseases, traumatic brain injury, and heart disease
- CC0000: Color is not claimed as a feature of the mark.
- GS0011: Biochemicals in the nature of synthetic RNA encoding natural or engineered nucleases, zinc-finger nucleases, meganucleases, nickases, and transcription activator-like effector nucleases for use in vivo and in vitro genetic engineering
- GS0421: Pharmaceutical research and development services; pharmaceutical research and development services in the field of production of cell therapies, with or without gene-editing and with or without mRNA engineering; pharmaceutical research and development services in the field of, gene editing, mRNA engineering, cell reprogramming and cell therapy development; scientific laboratory services, in the field of stem cell production; scientific laboratory services in the field of production of stem cells and converting non-pluripotent cells into pluripotent stem cells, with or without gene-editing; scientific laboratory services in the field of gene-editing
Case File Event Statements
-
7/12/2018 - 7 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
7/10/2018 - 7 years ago
1 - NEW APPLICATION ENTERED
Type: NWAP
-
7/13/2018 - 7 years ago
3 - NOTICE OF DESIGN SEARCH CODE E-MAILED
Type: MDSC
-
10/25/2018 - 7 years ago
4 - ASSIGNED TO EXAMINER
Type: DOCK
-
10/27/2018 - 7 years ago
5 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
10/27/2018 - 7 years ago
6 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
10/27/2018 - 7 years ago
7 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
2/12/2019 - 6 years ago
8 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
2/18/2019 - 6 years ago
9 - ASSIGNED TO LIE
Type: ALIE
-
2/19/2019 - 6 years ago
10 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
2/19/2019 - 6 years ago
11 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
4/10/2019 - 6 years ago
12 - FINAL REFUSAL WRITTEN
Type: CNFR
-
5/9/2019 - 6 years ago
16 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
5/9/2019 - 6 years ago
17 - NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED
Type: GNEN
-
5/9/2019 - 6 years ago
18 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
4/10/2019 - 6 years ago
13 - FINAL REFUSAL E-MAILED
Type: GNFR
-
4/10/2019 - 6 years ago
14 - NOTIFICATION OF FINAL REFUSAL EMAILED
Type: GNFN
-
5/9/2019 - 6 years ago
15 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
5/10/2019 - 6 years ago
19 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
5/29/2019 - 6 years ago
20 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
6/18/2019 - 6 years ago
22 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
6/18/2019 - 6 years ago
21 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
8/13/2019 - 6 years ago
23 - NOA E-MAILED - SOU REQUIRED FROM APPLICANT
Type: NOAM
-
12/18/2019 - 5 years ago
24 - TEAS STATEMENT OF USE RECEIVED
Type: EISU
-
2/5/2020 - 5 years ago
29 - NOTICE OF ACCEPTANCE OF STATEMENT OF USE E-MAILED
Type: SUNA
-
3/10/2020 - 5 years ago
30 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
12/18/2019 - 5 years ago
26 - USE AMENDMENT FILED
Type: IUAF
-
12/29/2019 - 5 years ago
27 - STATEMENT OF USE PROCESSING COMPLETE
Type: SUPC
-
12/29/2019 - 5 years ago
25 - CASE ASSIGNED TO INTENT TO USE PARALEGAL
Type: AITU
-
2/4/2020 - 5 years ago
28 - ALLOWED PRINCIPAL REGISTER - SOU ACCEPTED
Type: CNPR
-
3/10/2025 - 7 months ago
31 - COURTESY REMINDER - SEC. 8 (6-YR) E-MAILED
Type: REM1