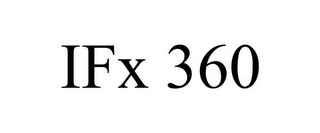Information
-
 Trademark
Trademark
-
 88081549
88081549
-
International Classifications
- 1 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry
- 5 - Pharmaceutical and veterinary preparations
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
- 10 - Surgical, medical, dental and veterinary apparatus and instruments, artificial limbs, eyes and teeth
-
Filing Date
August 16, 2018
7 years ago
-
Transaction Date
February 10, 2020
5 years ago
-
Status Date
February 10, 2020
5 years ago
-
Published for Opposition Date
May 14, 2019
6 years ago
-
Location Date
July 09, 2019
6 years ago
-
Status Code
606
-
Current Location
INTENT TO USE SECTION
Employee Name
PAQUIN, SAMUEL ROBERT
-
Attorney Docket Number
1092854
Attorney Name
Margaret C. McHugh
Law Office Assigned Location Code
L10
-
Owners
Mark Drawing Code
4000
Mark Identification
IFX 360
Case File Statements
- GS0011: Assays for research purposes; biological preparations for use in industry and science, for assay purposes; chemical preparations for scientific assay purposes, other than for medical or veterinary use; chemical reagents for assay purposes, other than for medical or veterinary purpose; chemical substances for analyses in laboratories, other than for medical or veterinary purposes; chemical test kits for diagnosis, prognosis, and monitoring of autoimmune diseases for laboratory or research use; diagnostic preparations for clinical or medical laboratory use; diagnostic reagents and preparations, except for medical or veterinary use; diagnostic reagents for clinical or medical laboratory use; diagnostic reagents for scientific or research use; diagnostic reagents for in vitro use in biochemistry, clinical chemistry and microbiology; laboratory chemicals, namely, an antibody reagent used for the detection of antigens in cell and tissue analysis for in vitro diagnostic use
- GS0051: Chemical reagents for medical or veterinary assay purposes; chemical and biological preparations for medical purposes, namely, for diagnosis, prognosis, and monitoring of autoimmune diseases; diagnostic agents, preparations and substances for medical purposes; diagnostic preparations for medical purposes; diagnostic preparations for medical purposes for detecting the presence of inflammatory and autoimmune disorders and conditions and tissue trauma in humans; diagnostic biomarker reagents for medical purposes; diagnostic kits consisting primarily of monoclonal antibodies, buffers, and reagents for use in disease testing; diagnostic reagents for medical use; diagnostic kits comprised of medical diagnostic reagents and assays for testing of bodily fluids for use in disease detection, namely, autoimmune diseases; medical diagnostic reagents; medical diagnostic reagents and assays for testing of body fluids; none of the aforesaid goods bearing therapeutics
- GS0091: Scientific instruments, namely, electronic analyzers for testing and analyzing chemical and biological substances for the presence, absence, or quantity of autoantibodies associated with autoimmune diseases; plates, glass slides with one or more sample areas that can be used in chemical analysis, biological analysis, or patterning for scientific, laboratory or medical research use; integrated computerized system for the collection, editing, organizing, modifying, bookmarking, transmission, storage and sharing of data and information related to chemical analyses; computer hardware and software, and diagnostic reagents and controls, for use in diagnosis, prognosis, and monitoring of autoimmune diseases
- GS0101: Medical apparatus and instruments for diagnostic use, namely, instruments for medical diagnostic testing in the field of autoimmune diseases; medical diagnostic instruments for the analysis of body fluids; medical diagnostic equipment for use in diagnosis, prognosis, and monitoring of autoimmune diseases, namely, an automated system consisting of computer-controlled electronic apparatus, computer hardware and software, and diagnostic reagents and controls, for use in diagnosis, prognosis, and monitoring of autoimmune diseases
- PM0001: IFX THREE HUNDRED SIXTY
Case File Event Statements
-
2/10/2020 - 5 years ago
17 - ABANDONMENT NOTICE E-MAILED - NO USE STATEMENT FILED
Type: MAB6
-
2/10/2020 - 5 years ago
16 - ABANDONMENT - NO USE STATEMENT FILED
Type: ABN6
-
7/9/2019 - 6 years ago
15 - NOA E-MAILED - SOU REQUIRED FROM APPLICANT
Type: NOAM
-
5/14/2019 - 6 years ago
14 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
5/14/2019 - 6 years ago
13 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
4/24/2019 - 6 years ago
12 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
3/31/2019 - 6 years ago
11 - ASSIGNED TO LIE
Type: ALIE
-
3/13/2019 - 6 years ago
10 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
3/12/2019 - 6 years ago
9 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
3/11/2019 - 6 years ago
8 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
3/11/2019 - 6 years ago
7 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
9/11/2018 - 7 years ago
6 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
9/11/2018 - 7 years ago
5 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
9/11/2018 - 7 years ago
4 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
9/6/2018 - 7 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
8/25/2018 - 7 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM
Type: NWOS
-
8/20/2018 - 7 years ago
1 - NEW APPLICATION ENTERED IN TRAM
Type: NWAP