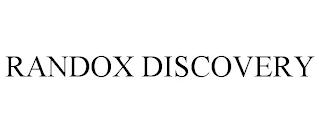Information
-
 Trademark
Trademark
-
 88318383
88318383
-
Serial Number
88318383
-
Registration Number
6203127
-
International Classifications
- 1 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry
- 5 - Pharmaceutical and veterinary preparations
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
- 10 - Surgical, medical, dental and veterinary apparatus and instruments, artificial limbs, eyes and teeth
-
Filing Date
February 27, 2019
5 years ago
-
Registration Date
November 24, 2020
4 years ago
-
Transaction Date
November 24, 2020
4 years ago
-
Status Date
November 24, 2020
4 years ago
-
Published for Opposition Date
January 14, 2020
5 years ago
-
Location Date
October 21, 2020
4 years ago
-
Status Code
700
-
Current Location
PETITIONS OFFICE
Employee Name
CIURPITA, DREW PETER F
-
Attorney Docket Number
210336-00035
Attorney Name
Daniel Frohling
Law Office Assigned Location Code
M50
-
Owners
Mark Drawing Code
4000
Mark Identification
RANDOX DISCOVERY
Case File Statements
- GS0011: Diagnostic preparations for scientific purposes; chemical reagents for scientific or medical research and for use in the analysis of food products; enzymes for use in scientific and medical research applications; protein arrays for use in scientific and medical research applications; diagnostic preparations for analysis of food products and water for scientific or research use; clinical diagnostic preparations for clinical or medical laboratory use; clinical diagnostic reagents for in vitro use for clinical or medical laboratory use; chemical reagents other than for medical or veterinary purposes, namely, chemical reagents for detection and monitoring of disease states in vitro for laboratory research purposes; diagnostic proteins, namely, protein arrays for scientific and medical research featuring antibodies and antigens used for monitoring of disease states in vitro
- GS0051: Diagnostic preparations and substances for medical and veterinary purposes; diagnostic kits in the nature of clinical chemistry and immunology test kits for medical use in the detection and monitoring of disease states in vitro, namely, chemical reagents for medical or veterinary purposes for the detection and monitoring of disease states including cardiac, DOA, stroke, metabolic, cancer, diabetes, fertility, thyroid and DNA arrays; clinical diagnostic preparations, for medical or veterinary use; clinical diagnostic reagents for in vivo use for medical or veterinary purposes; diagnostic scanning agents for in vivo use for medical or veterinary purposes; diagnostic testing materials for medical use in the detection and monitoring of disease states in vitro, namely, chemical reagents for medical or veterinary purposes for the detection and monitoring of disease states including cardiac, DOA, stroke, metabolic, cancer, diabetes, fertility, thyroid and DNA arrays; medical diagnostic proteins, namely, protein arrays for medical diagnostic purposes featuring antibodies and antigens used for medical detection of disease states in vitro; chemical reagents, namely, chemical reagents for detection and monitoring of disease states in vitro
- GS0091: Downloadable computer software for use in conjunction with in vitro diagnostic testing apparatus, namely, software for controlling medical and clinical sample processing, sample result reporting and test apparatus system robotics; computer hardware for or relating to the analysis, monitoring, research and/or reporting of clinical data; downloadable computer programs for use in conjunction with in vitro diagnostic testing apparatus for controlling medical and clinical sample processing and sample result reporting; computer systems, namely, computer hardware and downloadable software for use in conjunction with in vitro diagnostic testing apparatus for controlling medical and clinical sample processing and sample result reporting; computer systems for use in conjunction with in vitro diagnostic testing apparatus, namely, downloadable computer software and hardware for controlling medical and clinical sample processing; downloadable computer databases and computer databases recorded on computer media for use in connection with in vitro diagnostic testing apparatus, namely, downloadable electronic data files featuring medical diagnostic sample results; scientific and optical apparatus and instruments, namely, a benchtop analytical system composed of barcode scanner and associated software for reading barcodes, a chemiluminescent detection system in the nature of instruments for measuring chemiluminescence, a temperature controlled incubator for laboratory use, and an electronic reagent and patient sample dispenser, suitable for protein and DNA arrays for or relating to the analysis, monitoring, research and/or reporting of clinical data, for use in vitro diagnostic testing procedures for clinical, veterinary, food and toxicological analysis; clinical analyzers not for medical and veterinary use, namely, acidity analyzers, liquid analyzers, residual gas analyzers for use in vitro diagnostic testing procedures and for use in measuring blood proteins, antibodies, enzymes, immunoglobulins, hormones and drugs for laboratory use; light detectors for use in vitro diagnostic testing procedures, namely, a chemiluminescence detection system not for medical purposes; parts and fittings for all the aforesaid goods, namely, replacement parts and fittings
- GS0101: Medical apparatus and instruments for medical use, namely, for use in vitro diagnostic testing procedures to detect and diagnose drug residues used in immunoassay procedures and for use in measuring blood proteins, antibodies, enzymes, immunoglobulins and hormones; diagnostic apparatus and instruments for medical use, namely, for use in vitro diagnostic testing procedures to detect and diagnose drug residues used in immunoassay procedures and for use in measuring blood proteins, antibodies, enzymes, immunoglobulins, hormones and drugs; diagnostic imaging apparatus for medical use, namely, electromechanical diagnostic analyser utilizing a chemiluminescent detection system for use in in vitro diagnostic testing procedures and for use in measuring blood proteins, antibodies, enzymes, immunoglobulins, hormones and drugs; medical diagnostic testing apparatus and instruments for use in immunoassay procedures, namely, for use in the measurement of antibodies, immunoglobulins, hormones and drugs representing various drug classes; light detectors for use in vitro diagnostic testing procedures, namely, a chemiluminescence detection system for medical purposes; clinical analyzers for medical and veterinary use, namely, acidity analyzers, liquid analyzers, residual gas analyzers for use in vitro diagnostic testing procedures and for use in measuring blood proteins, antibodies, enzymes, immunoglobulins, hormones and drugs
Case File Event Statements
-
11/24/2020 - 4 years ago
29 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
10/22/2020 - 4 years ago
28 - TEAS RESPONSE TO PETITION TO DIRECTOR INQUIRY
Type: ERTI
-
10/21/2020 - 4 years ago
27 - PETITION TO DIRECTOR - CHANGE BASIS - GRANTED
Type: PCBG
-
10/20/2020 - 4 years ago
26 - ASSIGNED TO PETITION STAFF
Type: APET
-
10/14/2020 - 4 years ago
25 - EXTENSION RECEIVED WITH TEAS PETITION
Type: TPEX
-
10/13/2020 - 4 years ago
24 - ABANDONMENT NOTICE E-MAILED - NO USE STATEMENT FILED
Type: MAB6
-
10/12/2020 - 4 years ago
23 - ABANDONMENT - NO USE STATEMENT FILED
Type: ABN6
-
9/1/2020 - 4 years ago
22 - TEAS PETITION TO AMEND BASIS RECEIVED
Type: TPAD
-
3/10/2020 - 4 years ago
21 - NOA E-MAILED - SOU REQUIRED FROM APPLICANT
Type: NOAM
-
1/14/2020 - 5 years ago
20 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
1/14/2020 - 5 years ago
19 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
12/25/2019 - 5 years ago
18 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
12/6/2019 - 5 years ago
17 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
12/5/2019 - 5 years ago
16 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
12/5/2019 - 5 years ago
15 - NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED
Type: GNEN
-
12/5/2019 - 5 years ago
14 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
12/5/2019 - 5 years ago
13 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
10/22/2019 - 5 years ago
12 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
10/22/2019 - 5 years ago
11 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
10/15/2019 - 5 years ago
10 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
5/15/2019 - 5 years ago
9 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
5/15/2019 - 5 years ago
8 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
5/15/2019 - 5 years ago
7 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
5/8/2019 - 5 years ago
6 - ASSIGNED TO EXAMINER
Type: DOCK
-
3/19/2019 - 5 years ago
5 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM
Type: NWOS
-
3/11/2019 - 5 years ago
4 - APPLICANT AMENDMENT PRIOR TO EXAMINATION - ENTERED
Type: AMPX
-
3/11/2019 - 5 years ago
3 - ASSIGNED TO LIE
Type: ALIE
-
3/6/2019 - 5 years ago
2 - TEAS VOLUNTARY AMENDMENT RECEIVED
Type: PARI
-
3/2/2019 - 5 years ago
1 - NEW APPLICATION ENTERED IN TRAM
Type: NWAP