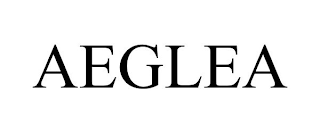Information
-
 Trademark
Trademark
-
 90138790
90138790
-
International Classifications
-
Filing Date
August 26, 2020
4 years ago
-
Transaction Date
August 17, 2024
5 months ago
-
Status Date
June 03, 2024
8 months ago
-
Published for Opposition Date
September 07, 2021
3 years ago
-
Location Date
November 02, 2021
3 years ago
-
Status Code
606
-
Current Location
INTENT TO USE SECTION
Employee Name
ASKEW, MEGAN RUTH
-
Attorney Docket Number
ABIO006US
Attorney Name
Christopher M. Kindel
Law Office Assigned Location Code
L80
-
Owners
Mark Drawing Code
4
Mark Identification
AEGLEA
Case File Statements
- GS0051: Pharmaceutical preparations for the treatment of rare genetic diseases; pharmaceutical preparations for the treatment of Arginase 1 deficiency; pharmaceutical preparations for the treatment of cancer; biopharmaceutical preparations for the treatment of rare genetic diseases; biopharmaceutical preparations for the treatment of Arginase 1 deficiency; biopharmaceutical preparations for the treatment of cancer; biotherapeutic preparations for the treatment of rare genetic diseases; biotherapeutic preparations for the treatment of Arginase 1 deficiency; biotherapeutic preparations for the treatment of cancer; biotherapeutic preparations and substances for the treatment of rare genetic diseases; biotherapeutic preparations and substances for the treatment of Arginase 1 deficiency; biotherapeutic preparations and substances for the treatment of cancer; enzyme replacement therapy preparations for medical purposes; pharmaceutical preparations for the prevention and treatment of infectious diseases and immunological diseases; biopharmaceutical preparations for the prevention and treatment of infectious diseases and immunological diseases; pharmaceutical preparations for the treatment of homocystinuria; biopharmaceutical preparations for the treatment of homocystinuria; biotherapeutic preparations for the treatment of homocystinuria
- GS0421: Scientific research in the fields of pharmaceuticals, biopharmaceuticals, medicine, biologics, biotherapeutics, biosimilars, enzyme replacement therapeutics, and therapeutics, all for the treatment of rare genetic diseases, Arginase 1 deficiency, cancer, immunological diseases, homocystinuria, and infectious diseases; medical and scientific research in the field of pharmaceutical and biopharmaceutical treatment of rare genetic diseases, Arginase 1 deficiency, cancer, immunological diseases, homocystinuria, and infectious diseases; providing medical and scientific research information, consultancy, and advisory services, all in the fields of pharmaceuticals, biopharmaceuticals, medicine, biologics, biotherapeutics, biosimilars, enzyme replacement therapeutics, and therapeutics treatments, and all for the treatment of rare genetic diseases, Arginase 1 deficiency, cancer, immunological diseases, homocystinuria, and infectious diseases; Design, engineering, research, development and testing services, all for medical research purposes in the fields of cancer, rare genetic disease, Arginase 1 deficiency treatments, immunological diseases, homocystinuria, and infectious diseases
Case File Event Statements
-
8/29/2020 - 4 years ago
1 - NEW APPLICATION ENTERED
Type: NWAP
-
9/29/2020 - 4 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
12/24/2020 - 4 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
6/30/2021 - 3 years ago
9 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
12/31/2020 - 4 years ago
4 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
12/31/2020 - 4 years ago
5 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
12/31/2020 - 4 years ago
6 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
6/29/2021 - 3 years ago
8 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
6/29/2021 - 3 years ago
7 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
7/31/2021 - 3 years ago
10 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
9/7/2021 - 3 years ago
12 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
8/18/2021 - 3 years ago
11 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
9/7/2021 - 3 years ago
13 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
11/2/2021 - 3 years ago
14 - NOA E-MAILED - SOU REQUIRED FROM APPLICANT
Type: NOAM
-
4/29/2022 - 2 years ago
15 - SOU TEAS EXTENSION RECEIVED
Type: EEXT
-
4/29/2022 - 2 years ago
16 - SOU EXTENSION 1 FILED
Type: EXT1
-
4/29/2022 - 2 years ago
17 - SOU EXTENSION 1 GRANTED
Type: EX1G
-
11/1/2022 - 2 years ago
21 - SOU EXTENSION 2 GRANTED
Type: EX2G
-
5/3/2022 - 2 years ago
18 - NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED
Type: EXRA
-
11/1/2022 - 2 years ago
19 - SOU TEAS EXTENSION RECEIVED
Type: EEXT
-
11/1/2022 - 2 years ago
20 - SOU EXTENSION 2 FILED
Type: EXT2
-
11/3/2022 - 2 years ago
22 - NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED
Type: EXRA
-
5/1/2023 - a year ago
23 - SOU TEAS EXTENSION RECEIVED
Type: EEXT
-
11/2/2023 - a year ago
28 - SOU EXTENSION 4 FILED
Type: EXT4
-
5/1/2023 - a year ago
24 - SOU EXTENSION 3 FILED
Type: EXT3
-
5/1/2023 - a year ago
25 - SOU EXTENSION 3 GRANTED
Type: EX3G
-
5/3/2023 - a year ago
26 - NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED
Type: EXRA
-
11/2/2023 - a year ago
27 - SOU TEAS EXTENSION RECEIVED
Type: EEXT
-
11/2/2023 - a year ago
29 - SOU EXTENSION 4 GRANTED
Type: EX4G
-
11/4/2023 - a year ago
30 - NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED
Type: EXRA
-
6/3/2024 - 8 months ago
32 - ABANDONMENT - NO USE STATEMENT FILED
Type: ABN6
-
5/16/2024 - 8 months ago
31 - ASSIGNED TO EXAMINER
Type: DOCK
-
7/12/2024 - 7 months ago
33 - ABANDONMENT NOTICE E-MAILED - NO USE STATEMENT FILED
Type: MAB6