Information
-
 Trademark
Trademark
-
 90741545
90741545
-
International Classifications
-
Filing Date
May 28, 2021
3 years ago
-
Transaction Date
March 25, 2024
a year ago
-
Status Date
March 25, 2024
a year ago
-
Published for Opposition Date
June 28, 2022
2 years ago
-
Location Date
August 23, 2022
2 years ago
-
Status Code
606
-
Current Location
INTENT TO USE SECTION
Employee Name
IN, SUNG HYUN
-
Attorney Docket Number
106517999102
Attorney Name
Carrie L. Kiedrowski
Law Office Assigned Location Code
L30
-
Owners
Case File Statements
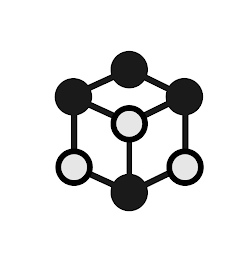
- DM0000: The mark consists of a hexagon design in which the top left, right and bottom center corners of the hexagon are connected by lines. The top left, center and right corners and the bottom center corner of the hexagon have an solid dot overlapping each corner. The bottom left and right corners of the hexagon have a hollow dot overlapping each corner. The center corner created by the intersecting lines also contains an overlapping hollow dot.
- GS0441: Providing genomic, imaging and pathology data being medical information and medical analyses based on collected clinical and research information in the field of cancer and other diseases for medical purposes; analysis of cells, tissues, gene expressions, gene sequences, genome annotation, transcriptome characterization, and genome mapping for medical diagnosis and treatment of cancer, neurological disease, respiratory disease, inflammatory disease, and mitochondrial disease; preparation of reports relating to gene expressions, gene and genome sequences, genome interaction and annotation, transcriptome analysis and characterization, and genome mapping for medical diagnosis and treatment of cancer, neurological disease, respiratory disease, inflammatory disease, and mitochondrial disease; medical assistance services in the nature of genetic and medical information provided to medical professionals from remote locations via the internet and global computer networks through the use of archived genome sequence information, medical images, diagnostic test results, and a data storage and retrieval system; medical genetic testing consultations provided via phone, online chat or videoconferencing; medical diagnostic testing, monitoring and reporting services; medical imaging services; medical information; medical screening; medical testing for diagnostic purposes; multi-disciplinary, integrative, genetic consultations for medical treatment purposes; providing a website featuring genetic and medical information; providing an internet website for medical professionals and medical patients featuring medical information from remote locations via devices that feed information to the website that is processed, exchanged and accessed in real-time by users; providing an internet website for medical professionals and medical patients featuring medical information from remote locations via electronic patient monitoring devices that feed information to the website that can be accessed in real-time by medical professionals for purposes of monitoring and diagnosing medical conditions; providing medical profiles and medical record analysis and assessments via a website that are designed to provide custom tailored outputs about recommended resources and treatments associated with a defined set of symptoms, concerns, genome sequencing analysis, microbiome sequencing analysis, metabolome analysis, advanced clinical imaging, laboratory tests, and medical imaging and DNA screening; providing medical testing and medical consultations to individuals and their physicians to help them make health, wellness and nutritional changes in their daily living to improve health; providing on-line medical record analysis services designed to provide patients with custom tailored information about the range of possible diagnoses and therapies associated with a defined set of symptoms based on genome sequencing analysis, microbiome sequencing analysis, metabolome analysis, advanced clinical imaging, laboratory tests, and medical imaging and DNA screening; provision of health care and medical services by health care professionals via the internet or telecommunication networks; remote monitoring of data indicative of the health or condition of an individual or group of individuals for medical diagnosis and treatment purposes; health assessment services, namely, providing assessment of risk of disease and providing health monitoring of individuals all through use of genome sequencing analysis and reporting, microbiome sequencing analysis, metabolome analysis, advanced clinical imaging, laboratory tests, and medical imaging and DNA screening; all for medical treatment and diagnostic purposes
- GS0421: Cloud computing featuring software for analyzing genomic, imaging, pathology and clinical data; consulting services relating to scientific and medical research concerning genomic, imaging, pathology and clinical data; providing temporary use of non-downloadable computer software for hosting, managing, sharing, transmitting, and analyzing patient genomic, imaging, pathology and clinical data to improve the quality of medical diagnosis, care, and outcome; design, development, computer programming, testing, maintenance, customization, and operation of computer software in clinical trials or studies; designing clinical trials or studies, enriching and enhancing clinical trial or study designs in the nature of research and development in the field of medicine; clinical research in the fields of de-risking clinical trial or study designs, clinical trials simulations and clinical endpoint selection; clinical research, namely, analyzing non-clinical data, preclinical data, and clinical data, analyzing results of clinical trials or studies, and evaluating clinical outcomes and treatment responses; providing scientific analysis in the field of next generation sequencing (NGS), namely, the analysis and interpretation of genomic, imaging, pathology and clinical data; medical and scientific research, namely, conducting laboratory testing for clinical trials of pharmaceuticals and medical devices in humans; health care services, namely, medical laboratory services and clinical scientific laboratory services for testing clinical specimens for the purpose of providing information for medical research, clinical trials, and pre-clinical investigations; providing a website featuring temporary use of non-downloadable software for clinical and safety information management, process facilitation for clinical endpoint adjudication, electronic data capture of member voting, efficient case packaging and automated databasing of voting and decision data for use in pharmaceutical or medical device clinical trials; clinical research in the fields of patient identification and a priori responder selection, individualizing treatments, predicting clinical outcomes and treatment responses, and disease diagnoses and treatment determination, selection, and identification, drug, drug target, and drug combination selection and evaluation, biomarker selection, genetic analyses, omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics analyses; clinical research, namely, accessing and analyzing omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics data, and in the medical field, biomedical field, healthcare field, pharmaceutical field, and biotechnological field using artificial intelligence, machine learning, supervised machine learning, unsupervised machine learning, deep learning, statistical learning, data mining, big data analytics, predictive analytics, predictive modeling, multivariate analytics, precision medicine, bioinformatics, modeling, and simulation; consulting, consultancy, and consulting services related to clinical trials or studies, namely, designing clinical trials or studies, enriching and enhancing clinical trial or study designs in the nature of research and development in the field of medicine, de-risking clinical trial or study designs, conducting clinical trials simulations, clinical endpoint selection, analyzing preclinical data, and clinical data, analyzing results of clinical trials or studies, and evaluating clinical outcomes and treatment responses; consulting, consultancy, and consulting services related to clinical trials or studies, namely, conducting patient identification and a priori responder selection, individualizing treatments, predicting clinical outcomes and treatment responses, and disease diagnoses and treatment determination; conducting drug, drug target, and drug combination selection and evaluation, biomarker selection, genetic analyses in the nature of medical research, and pharmaceutical development, in the fields of omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics analyses, and accessing, processing, and analyzing omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics data, all related to the medical field, biomedical field, healthcare field, pharmaceutical field, and biotechnological field using artificial intelligence, machine learning, supervised machine learning, unsupervised machine learning, deep learning, statistical learning, data mining, big data analytics, predictive analytics, predictive modeling, multivariate analytics, precision medicine, bioinformatics, modeling, and simulation; medical research in the nature of disease diagnoses and treatment determination, selection, and identification of cells, proteins, small molecules, and nucleotides in a sample for scientific, research, and technological purposes, drug, drug target, and drug combination selection and evaluation, biomarker selection, genetic analyses, omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics analyses, and accessing, processing, and analyzing omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics data, and services all in the medical field, biomedical field, healthcare field, pharmaceutical field, and biotechnological field using artificial intelligence, machine learning, supervised machine learning, unsupervised machine learning, deep learning, statistical learning, data mining, big data analytics, predictive analytics, predictive modeling, multivariate analytics, precision medicine, bioinformatics, modeling, and simulation for others; platform as a service (PaaS) featuring computer software platforms for hosting, managing, sharing, transmitting, and analyzing patient genomic, imaging, pathology and clinical data to improve the quality of medical diagnosis, care, and outcome; all of the foregoing in the field of clinical care, medical diagnostic, and scientific research services; platform as a service (PaaS) featuring computer software platforms for providing clinical decision support for patient care; platform as a service (PaaS) featuring computer platforms and software for using artificial intelligence for machine learning, supervised machine learning, unsupervised machine learning, deep learning, statistical learning, data mining, big data analytics, predictive analytics, predictive modeling, multivariate analytics for the analysis of cells, tissues, gene expression, gene sequences, genome annotation, transcriptome characterization and genome mapping for the purpose of medical research, and pharmaceutical development; platform as a service (PaaS) featuring computer platforms and software for using artificial intelligence for precision medicine, bioinformatics, modeling, and simulation in clinical trials or studies, designing clinical trials or studies, enriching and enhancing clinical trial or study designs in the nature of research and development in the field of medicine, de-risking clinical trial or study designs, clinical trials simulations, clinical endpoint selection, analyzing non-clinical data, preclinical data, and clinical data, analyzing results of clinical trials or studies, and evaluating clinical outcomes and treatment responses, patient identification and a priori responder selection, individualizing treatments, predicting clinical outcomes and treatment responses, and disease diagnoses and treatment determination, selection, and identification, drug, drug target, and drug combination selection and evaluation, biomarker selection, genetic analyses, omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics analyses, and accessing, processing, and analyzing omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics data, all in the medical field, biomedical field, healthcare field, pharmaceutical field, and biotechnological field; software as a service (SaaS) services featuring software for use in medical diagnostics, medical image analysis, medical screening, medical testing, DNA screening for medical purposes, and genetic testing for medical purposes; software as a service (SaaS) services featuring software for the analysis of medical data, namely, genomic, imaging, pathology and clinical data for use in the prevention, diagnosis and treatment of medical conditions, analyzing clinical trials, analyzing drug interactions, development of vaccines and medicines, analyzing medical diagnostic data, analyzing molecular medicine data, analyzing precision medicine data, and structural and functional analysis of genomes; software as a service (SaaS) services featuring software for the analysis of genomic data for use in tracking variation and mutation of pathogens; software as a service (SaaS) services featuring software for determining and predicting patterns in data, namely, medical data, genomic data, proteomic data, metabolomics data, transcriptomic data, nutritional and nutrigenomics data, medical imaging data, health care data, health records, medical records, genomic data, and environmental factor data; software as a service (SaaS) services featuring software for executing preconfigured non-field specific machine learning and deep learning algorithms and processes for the purposes of uploading and managing data for the training of preconfigured data models implemented on a hosted software platform utilizing said machine learning and deep learning processes and for utilizing said trained data models for use in medical diagnostics, medical image analysis, medical screening, medical testing, DNA screening for medical purposes, and genetic testing for medical purposes; software as a service (SaaS) services featuring software for conducting natural language processing on medical records to transform unstructured data into structured data supporting clinical and research use; software as a service (SaaS) featuring computer platforms for using artificial intelligence for determination, selection, and identification, drug, drug target, and drug combination selection and evaluation, biomarker selection, genetic analyses, omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics analyses, and accessing, processing, and analyzing omics, multiomics, genomics, transcriptomics, proteomics, lipidomics, and metabolomics data, all in the medical field, biomedical field, healthcare field, pharmaceutical field, and biotechnological field
Case File Event Statements
-
3/25/2024 - a year ago
34 - ABANDONMENT NOTICE E-MAILED - NO USE STATEMENT FILED
Type: MAB6
-
3/25/2024 - a year ago
33 - ABANDONMENT - NO USE STATEMENT FILED
Type: ABN6
-
10/26/2023 - a year ago
32 - ASSIGNED TO EXAMINER
Type: DOCK
-
6/15/2023 - a year ago
31 - NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED
Type: EXRA
-
6/13/2023 - a year ago
30 - SOU EXTENSION 2 GRANTED
Type: EX2G
-
6/13/2023 - a year ago
29 - SOU EXTENSION 2 FILED
Type: EXT2
-
6/13/2023 - a year ago
28 - SOU TEAS EXTENSION RECEIVED
Type: EEXT
-
1/26/2023 - 2 years ago
27 - NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED
Type: EXRA
-
1/24/2023 - 2 years ago
26 - SOU EXTENSION 1 GRANTED
Type: EX1G
-
1/24/2023 - 2 years ago
25 - SOU EXTENSION 1 FILED
Type: EXT1
-
1/24/2023 - 2 years ago
24 - SOU TEAS EXTENSION RECEIVED
Type: EEXT
-
8/23/2022 - 2 years ago
23 - NOA E-MAILED - SOU REQUIRED FROM APPLICANT
Type: NOAM
-
6/28/2022 - 2 years ago
22 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
6/28/2022 - 2 years ago
21 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
6/23/2022 - 2 years ago
20 - ASSIGNMENT OF OWNERSHIP NOT UPDATED AUTOMATICALLY
Type: ASCK
-
6/8/2022 - 2 years ago
19 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
5/25/2022 - 2 years ago
18 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
5/25/2022 - 2 years ago
17 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
5/25/2022 - 2 years ago
16 - NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED
Type: GNEN
-
5/25/2022 - 2 years ago
15 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
5/25/2022 - 2 years ago
14 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
5/9/2022 - 2 years ago
13 - NOTIFICATION OF FINAL REFUSAL EMAILED
Type: GNFN
-
5/9/2022 - 2 years ago
12 - FINAL REFUSAL E-MAILED
Type: GNFR
-
5/9/2022 - 2 years ago
11 - FINAL REFUSAL WRITTEN
Type: CNFR
-
4/5/2022 - 2 years ago
10 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
4/4/2022 - 2 years ago
9 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
4/4/2022 - 2 years ago
8 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
10/7/2021 - 3 years ago
7 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
10/7/2021 - 3 years ago
6 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
10/7/2021 - 3 years ago
5 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
10/6/2021 - 3 years ago
4 - ASSIGNED TO EXAMINER
Type: DOCK
-
8/24/2021 - 3 years ago
3 - NOTICE OF DESIGN SEARCH CODE E-MAILED
Type: MDSC
-
8/23/2021 - 3 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
6/1/2021 - 3 years ago
1 - NEW APPLICATION ENTERED
Type: NWAP