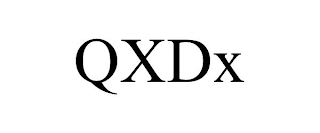Information
-
 Trademark
Trademark
-
 90775545
90775545
-
Serial Number
90775545
-
Registration Number
7139941
-
International Classifications
- 1 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry
- 5 - Pharmaceutical and veterinary preparations
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
- 10 - Surgical, medical, dental and veterinary apparatus and instruments, artificial limbs, eyes and teeth
-
Filing Date
June 15, 2021
4 years ago
-
Registration Date
August 15, 2023
2 years ago
-
Transaction Date
March 25, 2024
a year ago
-
Status Date
August 15, 2023
2 years ago
-
Published for Opposition Date
October 04, 2022
3 years ago
-
Location Date
July 13, 2023
2 years ago
-
First Use Anywhere Date
February 01, 2019
7 years ago
-
First Use In Commerce Date
February 01, 2019
7 years ago
-
Status Code
700
-
Current Location
PUBLICATION AND ISSUE SECTION
Employee Name
DEFORD, JEFFREY S
-
Attorney Docket Number
1254364
Attorney Name
Margaret C. McHugh
Law Office Assigned Location Code
M60
-
Owners
Mark Drawing Code
4000
Mark Identification
QXDX
Case File Statements
- GS0091: Apparatus, namely, medical laboratory research instruments and scientific laboratory research instruments for preparation, handling, amplification, and analysis of samples containing nucleic acids; accessories in the nature of laboratory apparatus, namely, sample preparation containers and kits consisting primarily of sample preparation containers and reagents, and instruction manuals sold as a unit therewith, used to prepare laboratory samples for use in scientific and medical research, all in connection with the preparation, handling, amplification, and analysis of samples containing nucleic acids; scientific apparatus, instruments and equipment for scientific research, non-medical diagnostic analysis, chemical analysis, clinical analysis and industrial use, namely, DNA and RNA testing apparatus; scientific laboratory equipment and apparatus, namely, digital polymerase chain reaction (dPCR) apparatus, electrophoresis apparatus, gel electrophoresis apparatus, and thermal cyclers, for use in amplifying, labeling, and analyzing nucleic acids, and parts and consumable parts therefor, namely, tubes, plates, cartridges, holders, and containers for liquids and gels; Laboratory apparatus and instruments, namely, digital Polymerase Chain Reaction (dPCR) instruments for amplifying DNA using digital Polymerase Chain Reaction (dPCR) for use in scientific research; Scientific apparatus and instruments, namely, droplet generators for sample preparation of nucleic acids and droplet reader for amplifying and analyzing samples containing nucleic acids; Downloadable and recorded software for use in for operation and maintenance of scientific apparatus, instruments and equipment, namely, operating quantitative Polymerase Chain Reaction (PCR) instruments; Polymerase Chain Reaction (PCR) instruments for use in scientific research, namely, laboratory apparatus and instruments for amplifying DNA using Polymerase Chain Reaction (PCR); downloadable computer software for use in amplifying, labeling, and analyzing nucleic acids; computer hardware and downloadable and integrated software for use in gene analysis); Downloadable and recorded computer software and hardware for processing diagnostic medical testing or laboratory data; Downloadable and recorded computer software and hardware for data analysis for use in laboratories; Downloadable and recorded computer software for operation and maintenance of scientific apparatus, instruments and equipment; Clinical medical laboratory apparatus and analytical instruments, namely, digital Polymerase Chain Reaction (dPCR) instruments for use in-vitro and in-vivo diagnostics
- GS0051: Diagnostic preparations for medical purposes; Diagnostic preparations for medical purposes, namely, reagents, enzymes, oligonucleotides, and buffer solutions for amplifying, labeling, and analyzing biopolymers and nucleic acids for diagnostic medical use and clinical use; Chemical reagents for medical or veterinary purposes; Chemical, biochemical and biotechnological preparations for medical and veterinary purposes, in particular reagents, enzymes and buffer solutions for marking, amplifying, quantification and/or for the analysis of biopolymers, in particular nucleic acids, in particular reagents, enzymes and buffer solutions for nucleic acid amplification; Medical diagnostic reagent kits comprised of clinical medical reagents and assays for clinical and medical use; Diagnostic test kits comprised of medical diagnostic reagents and assays for testing in the field of medical diagnostics; Kits comprising chemicals, reagents, enzymes, and buffer solutions for amplifying and labeling nucleic acids for diagnostic medical use and clinical use; Chemical test kits for cell assays for medical and veterinary use; Cartridges containing medical diagnostic reagents for use in Polymerase Chain Reaction (PCR) and digital Polymerase Chain Reaction (dPCR)
- PM0001: QXD X
- GS0011: Chemical reagents for scientific and medical research purposes; diagnostic reagents for clinical or medical laboratory use; Polymerase Chain Reaction (PCR) and digital Polymerase Chain Reaction (dPCR) reagents for scientific and medical research purposes; reagents for amplification and detection of nucleic acids for scientific and medical research purposes; Kits comprising chemicals, reagents, enzymes, and buffer solutions for amplifying and labeling nucleic acids for scientific and research use; Reagent kits comprising biological protein, DNA primers, polymerase or buffers for scientific and medical research use; Kits comprising chemicals, reagents, enzymes, and buffer solutions for amplifying and labeling nucleic acids for scientific or research use; Kits consisting primarily of reagents and magnetic, polymeric, or gel beads for scientific research use purposes
- GS0101: Apparatus, namely, DNA and RNA testing apparatus for medical and veterinary diagnostic analysis and determination of medical and veterinary conditions; Medical diagnostic apparatus and instruments, namely, Polymerase Chain Reaction (PCR) instrument to perform amplification and analyzation of nucleic acids; Medical devices and instruments, namely, a digital Polymerase Chain Reaction (dPCR) platform for use in measuring and quantifying copy number of DNA for medical diagnostic purposes; Medical devices and instruments, namely, a digital Polymerase Chain Reaction (dPCR) platform for use in measuring and quantifying copy number of DNA for medical diagnostic purposes; Diagnostic kits consisting primarily of electronic medical instruments for medical diagnosis and analysis of body fluids, tissues, biomolecules, DNA, antibodies, proteins, molecular material or cellular material
Case File Event Statements
-
8/15/2023 - 2 years ago
31 - NOTICE OF REGISTRATION CONFIRMATION EMAILED
Type: NRCC
-
8/15/2023 - 2 years ago
30 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
7/14/2023 - 2 years ago
29 - NOTICE OF ACCEPTANCE OF STATEMENT OF USE E-MAILED
Type: SUNA
-
7/13/2023 - 2 years ago
28 - ALLOWED PRINCIPAL REGISTER - SOU ACCEPTED
Type: CNPR
-
6/20/2023 - 2 years ago
27 - STATEMENT OF USE PROCESSING COMPLETE
Type: SUPC
-
5/26/2023 - 2 years ago
26 - USE AMENDMENT FILED
Type: IUAF
-
6/20/2023 - 2 years ago
25 - CASE ASSIGNED TO INTENT TO USE PARALEGAL
Type: AITU
-
5/26/2023 - 2 years ago
24 - TEAS STATEMENT OF USE RECEIVED
Type: EISU
-
11/29/2022 - 3 years ago
23 - NOA E-MAILED - SOU REQUIRED FROM APPLICANT
Type: NOAM
-
10/4/2022 - 3 years ago
22 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
10/4/2022 - 3 years ago
21 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
9/14/2022 - 3 years ago
20 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
8/26/2022 - 3 years ago
19 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
7/29/2022 - 3 years ago
18 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
7/29/2022 - 3 years ago
17 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
7/29/2022 - 3 years ago
16 - TEAS REQUEST FOR RECONSIDERATION RECEIVED
Type: ERFR
-
4/28/2022 - 3 years ago
15 - NOTIFICATION OF FINAL REFUSAL EMAILED
Type: GNFN
-
4/28/2022 - 3 years ago
14 - FINAL REFUSAL E-MAILED
Type: GNFR
-
4/28/2022 - 3 years ago
13 - FINAL REFUSAL WRITTEN
Type: CNFR
-
4/14/2022 - 3 years ago
12 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
4/13/2022 - 3 years ago
11 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
4/13/2022 - 3 years ago
10 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
11/4/2021 - 4 years ago
9 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
11/4/2021 - 4 years ago
8 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
11/4/2021 - 4 years ago
7 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
9/20/2021 - 4 years ago
6 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
9/20/2021 - 4 years ago
5 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
9/20/2021 - 4 years ago
4 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
9/20/2021 - 4 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
9/2/2021 - 4 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
6/18/2021 - 4 years ago
1 - NEW APPLICATION ENTERED
Type: NWAP