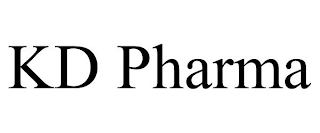Information
-
 Trademark
Trademark
-
 97376275
97376275
-
Serial Number
97376275
-
Registration Number
7593034
-
International Classifications
-
Filing Date
April 22, 2022
3 years ago
-
Registration Date
December 10, 2024
4 months ago
-
Transaction Date
December 10, 2024
4 months ago
-
Status Date
December 10, 2024
4 months ago
-
Published for Opposition Date
October 08, 2024
6 months ago
-
Location Date
December 09, 2024
4 months ago
-
Status Code
700
-
Current Location
FILE REPOSITORY (FRANCONIA)
Employee Name
KEELEY, ALISON
-
Attorney Docket Number
1986314
Attorney Name
KLAUS P. STOFFEL
-
Owners
Mark Drawing Code
4
Mark Identification
KD PHARMA
Case File Statements
- GS0291: Oils and fats, namely edible oils and edible fats and oils and fats for food; dietetic oils and fats in the nature of edible oils and fats, not for medical purposes; any cannabis or cannabis derived ingredients in the goods being solely derived from hemp with a delta-9 tetrahydrocannabinol (THC) concentration of not more than 0.3 percent on a dry weight basis; none of the foregoing containing cannabidiol (CBD)
- GS0051: Pharmaceutical, medical and veterinary preparations, namely, pharmaceutical preparations and substances for the treatment of infectious diseases, blood disorders, pain, inflammation, sepsis, alopecia, obesity and cognitive disorders, pharmaceutical products for the prevention and treatment of cancer, pharmaceutical preparations and substances for the treatment of viral, metabolic, endocrine, musculoskeletal, cardiovascular, cardiopulmonary, genitourinary, sexual dysfunction, oncological, hepatological, ophthalmic, respiratory, neurological, gastrointestinal, hormonal, dermatological, psychiatric and immune system related diseases and disorders, pharmaceutical preparations for the prevention and treatment of ocular disorders or diseases, for the treatment of bacteria-based diseases, and for the treatment of diabetes, and anti-infective preparations, antiviral preparations, antibiotics, antifungal preparations and vaccines, and chemical reagents for medical or veterinary purposes, chemical reagents for medical or veterinary purposes; sanitary preparations for medical purposes; dietetic foodstuffs and goods for medical or veterinary purposes, namely, dietetic foods and beverages adapted for medical use, dietetic foods adapted for veterinary use, dietetic beverages adapted for veterinary use, and dietetic preparations adapted for medical purposes; food supplements for humans and animals; disinfectants; medicines and natural remedies, namely medicinal oils and antiallergic medicines, medicinal preparations for the treatment of infectious diseases and for use in oncology, medicinal drinks, medicinal preparations for infectious diseases, blood disorders, pain, inflammation, sepsis, alopecia, obesity and cognitive disorders, for the prevention and treatment of cancer, for the treatment of viral, metabolic, endocrine, musculoskeletal, cardiovascular, cardiopulmonary, genitourinary, sexual dysfunction, oncological, hepatological, ophthalmic, respiratory, neurological, gastrointestinal, hormonal, dermatological, psychiatric and immune system related diseases and disorders, for the prevention and treatment of ocular disorders or diseases, for the treatment of bacteria- based diseases, for the treatment of epilepsy, pain, anxiety, depression, sleep disorders, Parkinson's disease, nausea and vomiting, food disorders, arthritis, PTSD, schizophrenia, opioid addiction, alleviate ALS symptoms, multiple sclerosis, and for the treatment of diabetes, natural remedy preparations for the treatment of gastro-intestinal conditions, hormonal and chemical imbalances, and sleep disorders; dietetic preparations, namely Dietetic preparations adapted for medical purposes; food supplements; food for babies; food for infants; infant formula; any cannabis or cannabis derived ingredients in the goods being solely derived from hemp with a delta-9 tetrahydrocannabinol (THC) concentration of not more than 0.3 percent on a dry weight basis; none of the foregoing containing cannabidiol (CBD)
- GS0011: Oils and fats, namely, lipids used in the manufacture of cosmetics, beverages, food products, pharmaceuticals and dietary supplements for the manufacture of pharmaceuticals, medical and veterinary preparations, sanitary preparations for medical purposes, dietetic foodstuffs and products for medical or veterinary purposes, food supplements for humans and animals, medicines and natural remedies, dietetic preparations and food supplements and pharmaceutical preparations and substances, dietary supplements and dietetic preparations, food for babies and infants, and for manufacturing beverages for infants and infant formula; any cannabis or cannabis derived ingredients in the goods being solely derived from hemp with a delta-9 tetrahydrocannabinol (THC) concentration of not more than 0.3 percent on a dry weight basis; none of the foregoing containing cannabidiol (CBD)
- DS0000: "PHARMA"
Case File Event Statements
-
4/26/2022 - 2 years ago
1 - NEW APPLICATION ENTERED
Type: NWAP
-
4/27/2022 - 2 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
2/9/2023 - 2 years ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
2/18/2023 - 2 years ago
4 - ASSIGNED TO EXAMINER
Type: DOCK
-
3/1/2023 - 2 years ago
5 - ASSIGNED TO EXAMINER
Type: DOCK
-
3/20/2023 - 2 years ago
6 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
3/20/2023 - 2 years ago
7 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
3/20/2023 - 2 years ago
8 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
6/19/2023 - a year ago
9 - APPLICATION EXTENSION TO RESPONSE PERIOD - RECEIVED
Type: XELR
-
6/19/2023 - a year ago
10 - APPLICATION EXTENSION GRANTED/RECEIPT PROVIDED
Type: XELG
-
9/20/2023 - a year ago
11 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
9/20/2023 - a year ago
12 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
9/21/2023 - a year ago
13 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
12/4/2023 - a year ago
14 - FINAL REFUSAL WRITTEN
Type: CNFR
-
12/4/2023 - a year ago
15 - FINAL REFUSAL E-MAILED
Type: GNFR
-
12/4/2023 - a year ago
16 - NOTIFICATION OF FINAL REFUSAL EMAILED
Type: GNFN
-
3/4/2024 - a year ago
17 - APPLICATION EXTENSION TO RESPONSE PERIOD - RECEIVED
Type: XELR
-
3/4/2024 - a year ago
18 - APPLICATION EXTENSION GRANTED/RECEIPT PROVIDED
Type: XELG
-
6/4/2024 - 10 months ago
22 - TEAS REQUEST FOR RECONSIDERATION RECEIVED
Type: ERFR
-
6/4/2024 - 10 months ago
19 - EXPARTE APPEAL RECEIVED AT TTAB
Type: EXAF
-
6/4/2024 - 10 months ago
21 - EX PARTE APPEAL-INSTITUTED
Type: EXPI
-
6/4/2024 - 10 months ago
20 - JURISDICTION RESTORED TO EXAMINING ATTORNEY
Type: JURT
-
7/1/2024 - 9 months ago
23 - SUBSEQUENT FINAL REFUSAL WRITTEN
Type: CFRC
-
7/1/2024 - 9 months ago
24 - SUBSEQUENT FINAL EMAILED
Type: GNSF
-
7/1/2024 - 9 months ago
25 - NOTIFICATION OF SUBSEQUENT FINAL EMAILED
Type: GNS1
-
9/4/2024 - 7 months ago
27 - EXAMINERS AMENDMENT -WRITTEN
Type: CNEA
-
9/4/2024 - 7 months ago
30 - EXAMINER'S AMENDMENT ENTERED
Type: XAEC
-
9/4/2024 - 7 months ago
29 - NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED
Type: GNEN
-
9/4/2024 - 7 months ago
28 - EXAMINERS AMENDMENT E-MAILED
Type: GNEA
-
9/4/2024 - 7 months ago
31 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
9/4/2024 - 7 months ago
26 - JURISDICTION RESTORED TO EXAMINING ATTORNEY
Type: JURT
-
9/4/2024 - 7 months ago
32 - EXPARTE APPEAL TERMINATED
Type: EXPT
-
9/18/2024 - 7 months ago
33 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
10/8/2024 - 6 months ago
34 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
10/8/2024 - 6 months ago
35 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
12/10/2024 - 4 months ago
36 - REGISTERED-PRINCIPAL REGISTER
Type: R.PR
-
12/10/2024 - 4 months ago
37 - NOTICE OF REGISTRATION CONFIRMATION EMAILED
Type: NRCC