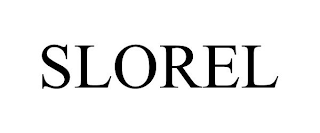Information
-
 Trademark
Trademark
-
 97593502
97593502
-
International Classifications
-
Filing Date
September 15, 2022
2 years ago
-
Transaction Date
January 09, 2025
25 days ago
-
Status Date
January 08, 2025
26 days ago
-
Location Date
January 08, 2025
26 days ago
-
Status Code
641
-
Current Location
TMO LAW OFFICE 112
Employee Name
SAXTON, EVELYN
-
Attorney Docket Number
8772CG-2
Attorney Name
Caroline E. Bryce
Law Office Assigned Location Code
M30
-
Owners
Mark Drawing Code
4
Mark Identification
SLOREL
Case File Statements
- GS0051: Surgical implants comprised of living tissues for guided tissue regeneration; implants comprising living tissue; implantable materials for use in guided tissue regeneration in the nature of implants comprising living tissue; Surgical implants comprised of living tissues used in transplants, namely, vascular grafts being living tissue and human allograft tissue; surgical implants comprised of living tissues; human allograft tissue; bone void fillers comprised of living tissues; Stem cells for medical purposes, namely, surgical implants grown from stem cells; pharmaceutical preparations for use in tissue transplantation; tissue-regenerative pharmaceutical preparations; medicines for human purposes, namely, pharmaceutical preparations for the treatment of musculo-skeletal disorders; medicines for human purposes, namely, a drug delivery system comprising liquids, powders, tablets or capsules for the continuous release of a wide variety of therapeutic agents, namely, pharmaceutical preparations for the treatment of musculo-skeletal disorders; drugs for medical purposes, namely, drugs for use in bioregenerative procedures, drugs for use for regenerative purpose, drugs for use to assist with artificial bone growth, drugs for use in accelerating bone formation, drugs for use to stimulate new bone formation; pharmaceutical agents affecting sensory organs; pharmaceutical preparations for the prevention of diseases of the musculo- skeletal system; pharmaceutical preparations for the treatment of Musculo-skeletal disorders; cellular function activating agents for medical purposes; pharmaceutical preparations for use in organ transplantation; biological tissue cultures for medical purposes; drug delivery agents in the form of liquid, powders, tablets and capsules that provide controlled release of the active ingredients for a wide variety of pharmaceuticals; drug delivery agents in the form of dissolvable films added to bone substitutes; drug delivery agents in the form of dissolvable films for use in bioregenerative procedures; drug delivery agents in the form of dissolvable films that provide sustained release of drugs for use with implants and bone substitutes; drug delivery agents in the form of drug delivery compounds that facilitate sustained or extended release of the active ingredients for a wide variety of pharmaceuticals; drug delivery agents in the form of dissolvable films with controlled timing and rate of drug release; medicines for human use, namely pharmaceutical preparations for the treatment of musculo-skeletal disorders featuring controlled timing and rate of release; medical bone graft substitute, namely, biological tissue bone grafts; medical bone graft materials, namely, bone fillers consisting of living materials; medical bone graft materials with drug release function being bone growth media consisting of biological materials for medical bone graft purposes with drug release function; medical bone graft materials, namely, biological bone tissue intended for subsequent implantation in a bone graft with drug release function, that provide sustained release of drugs for use with implants and bone substitutes; medical bone graft materials being biological bone tissue intended for subsequent implantation with drug release function that are sold filled with pharmaceutical preparations used with implants and bone substitutes
Case File Event Statements
-
9/19/2022 - 2 years ago
1 - NEW APPLICATION ENTERED
Type: NWAP
-
10/1/2022 - 2 years ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
3/14/2023 - a year ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
4/14/2023 - a year ago
4 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
4/14/2023 - a year ago
5 - NON-FINAL ACTION E-MAILED
Type: GNRT
-
4/14/2023 - a year ago
6 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
5/12/2023 - a year ago
7 - TEAS RESPONSE TO OFFICE ACTION RECEIVED
Type: TROA
-
5/12/2023 - a year ago
8 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
5/12/2023 - a year ago
9 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
6/16/2023 - a year ago
10 - SUSPENSION LETTER WRITTEN
Type: CNSL
-
6/16/2023 - a year ago
11 - LETTER OF SUSPENSION E-MAILED
Type: GNSL
-
6/16/2023 - a year ago
12 - NOTIFICATION OF LETTER OF SUSPENSION E-MAILED
Type: GNS3
-
1/17/2024 - a year ago
13 - SUSPENSION CHECKED - TO ATTORNEY FOR ACTION
Type: RCCK
-
3/12/2024 - 10 months ago
14 - REPORT COMPLETED SUSPENSION CHECK CASE STILL SUSPENDED
Type: RCSC
-
8/13/2024 - 5 months ago
15 - TEAS RESPONSE TO SUSPENSION INQUIRY RECEIVED
Type: ERSI
-
11/5/2024 - 3 months ago
16 - ASSIGNED TO LIE
Type: ALIE
-
11/6/2024 - 2 months ago
18 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
11/6/2024 - 2 months ago
17 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
11/6/2024 - 2 months ago
22 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
11/6/2024 - 2 months ago
20 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
11/6/2024 - 2 months ago
21 - TEAS WITHDRAWAL AS DOMESTIC REPRESENTATIVE RECEIVED
Type: EWOR
-
11/6/2024 - 2 months ago
23 - TEAS WITHDRAWAL OF ATTORNEY RECEIVED-FIRM RETAINS
Type: EWAF
-
11/6/2024 - 2 months ago
19 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
11/12/2024 - 2 months ago
24 - CORRESPONDENCE RECEIVED IN LAW OFFICE
Type: CRFA
-
11/12/2024 - 2 months ago
25 - TEAS/EMAIL CORRESPONDENCE ENTERED
Type: TEME
-
12/12/2024 - a month ago
28 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
12/12/2024 - a month ago
29 - TEAS WITHDRAWAL OF ATTORNEY RECEIVED-FIRM RETAINS
Type: EWAF
-
12/12/2024 - a month ago
26 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
12/12/2024 - a month ago
27 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
12/17/2024 - a month ago
30 - APPLICANT/CORRESPONDENCE CHANGES (NON-RESPONSIVE) ENTERED
Type: CHAN
-
1/8/2025 - 26 days ago
33 - NOTIFICATION OF NON-FINAL ACTION E-MAILED
Type: GNRN
-
1/8/2025 - 26 days ago
31 - NON-FINAL ACTION WRITTEN
Type: CNRT
-
1/8/2025 - 26 days ago
32 - NON-FINAL ACTION E-MAILED
Type: GNRT