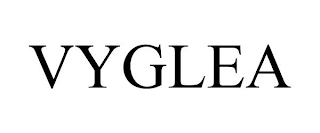Information
-
 Trademark
Trademark
-
 98042127
98042127
-
International Classifications
-
Filing Date
June 14, 2023
a year ago
-
Transaction Date
July 29, 2024
7 months ago
-
Status Date
July 29, 2024
7 months ago
-
Published for Opposition Date
October 31, 2023
a year ago
-
Location Date
December 26, 2023
a year ago
-
Status Code
606
-
Current Location
INTENT TO USE SECTION
Employee Name
FOSTER, ALEXANDRA M.
-
Attorney Docket Number
4501.0070001
Attorney Name
Monica Riva Talley
Law Office Assigned Location Code
M80
-
Owners
Mark Drawing Code
4
Mark Identification
VYGLEA
Case File Statements
- GS0051: Medicated and pharmaceutical preparations, both prescription and over-the-counter, for use in connection with humans and animals, namely, for the treatment of central nervous system disorders, neurologic illnesses, and psychiatric illnesses; pharmaceutical preparations for the treatment of brain ischemia; pharmaceutical preparations for the treatment of brain ischemia, namely, post-stroke recovery, severe intracranial atherosclerosis, carotid endarterectomy, cardiac bypass, cerebral vasculitis, reversible cerebral vasoconstriction syndrome; pharmaceutical preparations for the treatment of brain and cord injuries; pharmaceutical preparations for the treatment of brain and cord injuries, namely, post-intracranial hemorrhage recovery, traumatic brain injury, spinal cord injury; pharmaceutical preparations for the treatment of neurodegenerative diseases; pharmaceutical preparations for the treatment of neurodegenerative diseases, namely, Alzheimer's disease (AD), Amyotrophic Lateral Sclerosis (ALS), Huntington's disease, Lewy body dementia, frontotemporal dementia, vascular dementia, Parkinson's disease, cognitive impairment of aging, progressive Multiple Sclerosis; pharmaceutical preparations for the treatment of optic and retinal neuropathies; pharmaceutical preparations for the treatment of optic and retinal neuropathies, namely, glaucoma, diabetic retinopathy, ischemic optic and retinal neuropathies; pharmaceutical preparations for the treatment of pain; pharmaceutical preparations for the treatment of pain, namely, opioid sparing analgesia, cancer pain, chronic neuropathic pain, peripheral neuropathy, low back pain in the nature of failed back surgery syndrome, radiculopathy in the nature of sciatica, diabetic peripheral neuropathy, post-herpetic neuralgia, chemotherapy induced neuropathy and other medication induced neuropathies, complex regional pain syndrome, fibromyalgia, chronic post-operative pain, cluster thoracotomy, phantom limb, stump neuroma; pharmaceutical preparations for the treatment of headache and facial pain, namely, headache, migraine, chronic daily headache, cluster headache, trigeminal neuralgia; pharmaceutical preparations for symptomatic treatments of degenerative diseases; pharmaceutical preparations for symptomatic treatments of degenerative diseases, namely, breath holding in Rett syndrome, dystonias, dysphagia, gait abnormalities, bulbar function in ALS, pseudobulbar affect, levodopa-induced dyskinesias in Parkinson's disease, agitation in AD, disinhibition in dementia; pharmaceutical preparations for the treatment of epilepsy; pharmaceutical preparations for the treatment of epilepsy, namely, precision medicine - adjuvant for genetic epilepsy due to NMDA receptor mutations with hyperactivation; pharmaceutical preparations for the treatment of neuropsychiatric disorders; pharmaceutical preparations for the treatment of neuropsychiatric disorders, namely, treatment resistant depression, Post Traumatic Stress Disorder (PTSD) in the nature of hyperarousal, substance abuse disorders, addiction disorders, Obsessive Compulsive Disorder (OCD), hoarding, trichotillomania, eating disorders, Generalized Anxiety Disorder (GAD), adjustment disorders, panic disorder, major depressive episode, bipolar disorder in the nature of manic and depressive episodes, dysthymia, cyclothymia, psychotic disorders, schizophrenia; pharmaceutical preparations for the treatment of cerebellar disorders; pharmaceutical preparations for the treatment of cerebellar disorders, namely, spinocerebellar ataxia, Friedriech's ataxia, multisystem atrophy, essential tremor; pharmaceutical preparations for the treatment of hearing disorders, tinnitus, delirium, sleep disorders, parasomnias, learning disorders, Attention Deficit Disorder (ADD), Pervasive Developmental Disorders (PDD), Asperger's syndrome, autism, menopausal hot flashes; pharmaceutical preparations for the treatment of cancer; pharmaceutical preparations for the treatment of cancer, namely, monotherapy and adjunctive use
Case File Event Statements
-
6/17/2023 - a year ago
1 - NEW APPLICATION ENTERED
Type: NWAP
-
6/21/2023 - a year ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
9/23/2023 - a year ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
9/25/2023 - a year ago
4 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
10/11/2023 - a year ago
5 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
10/31/2023 - a year ago
6 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
10/31/2023 - a year ago
7 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
12/26/2023 - a year ago
8 - NOA E-MAILED - SOU REQUIRED FROM APPLICANT
Type: NOAM
-
1/5/2024 - a year ago
9 - ASSIGNED TO EXAMINER
Type: DOCK
-
7/29/2024 - 7 months ago
10 - ABANDONMENT - NO USE STATEMENT FILED
Type: ABN6
-
7/29/2024 - 7 months ago
11 - ABANDONMENT NOTICE E-MAILED - NO USE STATEMENT FILED
Type: MAB6