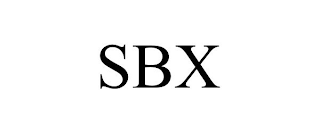Information
-
 Trademark
Trademark
-
 98212299
98212299
-
International Classifications
- 1 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry
- 5 - Pharmaceutical and veterinary preparations
- 9 - Scientific, nautical, surveying, photographic, cinematographic, optical, weighing, measuring, signalling, checking (supervision), life-saving and teaching apparatus and instruments
-
Filing Date
October 06, 2023
a year ago
-
Transaction Date
October 16, 2024
4 months ago
-
Status Date
October 15, 2024
4 months ago
-
Published for Opposition Date
April 30, 2024
10 months ago
-
Location Date
June 25, 2024
8 months ago
-
Status Code
730
-
Current Location
INTENT TO USE SECTION
Employee Name
ALI, STEPHANIE
-
Attorney Name
Mona Gupta
Law Office Assigned Location Code
L90
-
Owners
Mark Drawing Code
4
Mark Identification
SBX
Case File Statements
- GS0091: Scientific equipment, apparatus and instruments, namely, genome sequencer for scientific, pharmaceutical and medical research purposes; scientific instruments for use in scientific research, namely, nucleic acid sequencers, imaging devices and analyzers; laboratory apparatus for sample preparation, amplification, mixing, hybridization, incubation, and washing, namely, arrays, beads, bead sets and flow cells; laboratory equipment, namely, devices for analysis of nucleic acids and for dna sequencing; automated laboratory apparatus and systems, namely, sample loaders and bar code readers; downloadable software used for genotyping and gene expression microarrays for scientific, pharmaceutical and medical research purposes; downloadable software used in analyzing, managing and visualizing dna sequencing data; downloadable software used for preparation of nucleic acids libraries; computer hardware and downloadable software for collecting, storing, analyzing and reporting biological information, and for sample tracking and managing projects, laboratory workflow and data, for use in the fields of scientific, diagnostic and clinical research and for clinical diagnostic purposes
- GS0051: In vitro diagnostic agents, namely, reagents and control solutions for analysis of biological samples, all for medical diagnostic purposes; reagents for medical diagnostic use; medical diagnostic assays and reagents for testing of biological samples; reagent kits consisting of enzymes, oligonucleotides, nucleic acids, natural and modified nucleotides, buffers, labels, and substrates for medical diagnostic purposes; medical diagnostic test kits comprising reagents for testing body fluids to determine fetal chromosomal abnormalities; chemical reagents, preparations and test kits for fetal nucleic acid analysis for medical use; diagnostic kits consisting primarily of reagents for nucleic acid detection and analysis for medical diagnostic purposes; reagents for medical diagnostic use and diagnostic kits comprised of medical diagnostic reagents and assays for use in connection with dna sequencing and genetic analysis
- GS0011: In vitro diagnostic agents, namely, chemical control solutions for calibrating and monitoring the accuracy and function of instruments and test results, chemical additives, enzymes, reagents, organisms and proteins for use in science, research and industry; reagents for cell separation for scientific or research purposes; labeling reagents for scientific or research purposes; reagents for the processing, separation, isolation, enrichment, depletion, detection, screening, cultivation, analysis and counting of material, in particular of chemicals and biological material, all for scientific or research purposes; reagents featuring magnetic beads for scientific or research purposes; antibody reagents for scientific or research purposes; buffer solutions and reagents for use with laboratory apparatus, namely, separation apparatus and analysis apparatus, for scientific or research purposes; reagents for processing biological material, namely, tissue, bone marrow, cells, blood and components thereof for scientific or research purposes, all of the foregoing excluding reagents used in connection with demineralized bone for purposes other than diagnostic applications; reagents, enzymes, and nucleotides for nucleic acid sequencing for scientific or research purposes; diagnostic tests comprised of reagents and chemical test kits for determination of genetic information for scientific or research purposes; reagents for scientific or medical research use for analyzing cells, proteins, nucleic acids, sequencing dna, genotyping, gene expression profiling and high through-put screening; reagent kits composed primarily of oligonucleotides, enzymes and buffers for nucleic acid detection in the fields of scientific, pharmaceutical and medical research; nucleic acids, enzymes, buffers and chemical reagents for use in genotyping and gene expression test kits for detecting and analyzing nucleic acids for scientific or research purposes; assays and reagents for scientific or medical research use; diagnostic tests and test kits primarily composed of reagents and nucleotides for determination of genetic information for scientific or research purposes; reagents and chemical test kits for scientific and research use in connection with dna sequencing and genetic analysis
Case File Event Statements
-
10/10/2023 - a year ago
1 - NEW APPLICATION ENTERED
Type: NWAP
-
10/14/2023 - a year ago
2 - NEW APPLICATION OFFICE SUPPLIED DATA ENTERED
Type: NWOS
-
3/23/2024 - 11 months ago
3 - ASSIGNED TO EXAMINER
Type: DOCK
-
3/23/2024 - 11 months ago
4 - APPROVED FOR PUB - PRINCIPAL REGISTER
Type: CNSA
-
4/10/2024 - 11 months ago
5 - NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED
Type: NONP
-
4/30/2024 - 10 months ago
7 - OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED
Type: NPUB
-
9/10/2024 - 6 months ago
11 - TEAS CHANGE OF CORRESPONDENCE RECEIVED
Type: TCCA
-
4/30/2024 - 10 months ago
6 - PUBLISHED FOR OPPOSITION
Type: PUBO
-
6/25/2024 - 8 months ago
8 - NOA E-MAILED - SOU REQUIRED FROM APPLICANT
Type: NOAM
-
9/10/2024 - 6 months ago
9 - TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED
Type: REAP
-
9/10/2024 - 6 months ago
10 - ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED
Type: ARAA
-
10/15/2024 - 4 months ago
12 - SOU TEAS EXTENSION RECEIVED
Type: EEXT
-
10/15/2024 - 4 months ago
15 - NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED
Type: EXRA
-
10/15/2024 - 4 months ago
13 - SOU EXTENSION 1 FILED
Type: EXT1
-
10/15/2024 - 4 months ago
14 - SOU EXTENSION 1 GRANTED
Type: EX1G